3039
Resolving spin-spin couplings in hyperpolarized in vivo metabolic 13C spectroscopy at low magnetic field following murine tail-vein injection1Radiology, Vanderbilt University Institute of Imaging Science, Nashville, TN, United States
Synopsis
We demonstrate murine whole-body MRS and the ability to resolve the 13C multiplet of hyperpolarized 1-13C-succinate-d2 in a biplanar magnet with B0 = 0.0487 T and inhomogeneity <13 ppm over 40 cm DSV. At low magnetic field strength no loss of SNR relative to high field for a well-designed radiofrequency coil occurs, but chemical shift dispersion is potentially insufficient to differentiate hyperpolarized metabolites and contrast agents. However, at sufficiently low field strength, magnetic susceptibility derived B0 field inhomogeneity in vivo becomes negligible. Consequently, direct spectroscopic resolution of spin-spin couplings or J-couplings, more commonly performed near zero field, becomes feasible.
Overview
We report on work involving low-field in vivo MR detection at B0 = 0.0487 T of a hyperpolarized (HP) contrast agent (HCA) injected via tail-vein into a healthy wild-type mouse. At low magnetic field strengths there is no loss of SNR relative to high field for a well-designed radiofrequency (rf) coil,1,2 and the chemical shift dispersion is potentially insufficient to differentiate hyperpolarized (HP) metabolites and contrast agents. However, if the magnetic field is sufficiently low, then magnetic susceptibility derived B0 field inhomogeneity in vivo (the source of broad MRS lines at high field) becomes negligible. Consequently, direct spectroscopic resolution of spin-spin couplings or J-couplings, more commonly performed near zero field,3 becomes feasible. Here we demonstrate with whole-body detection for a mouse the ability to resolve the 13C multiplet of HP 1-13C-succinate-d2 (13C-SUX) in a magnet with B0 = 0.0487 T and inhomogeneity < 13 ppm over a 40 cm diameter spherical volume.Purpose
When injected into living organisms, hyperpolarized biomolecules can serve as metabolic contrast agents reporting on abnormal metabolism in cancer, heart diseases and other diseases and function as quantitative imaging biomarkers for these diseases. For example, efficient production of HP 13C-SUX enables one to probe in vivo mechanisms and pathways for cancer imaging4 using molecular imaging and spectroscopy.5Methods and Results
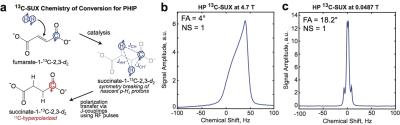
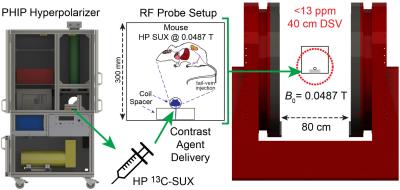
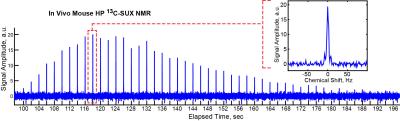
An open-source 13C PHIP hyperpolarizer6 (production cycle of 1 dose every 3 min, up to 28% 13C-SUX polarization) was used to produce hyperpolarized 13C-SUX contrast agent. Figure 1 shows the PHIP chemistry for production of 13C-SUX and comparison of shimmed MRS at field strengths of B0 = 4.7 T and B0 = 0.0487 T. The presence of foam in the phantom illustrates the difficulties of susceptibility-induced magnetic field B0 inhomogeneity at high fields. For the in vivo experiment, Figure 2, approximately 0.2 mL solution with ∼30 mM concentration in D2O of 13C-SUX was slowly injected via tail vein into a catheterized mouse situated inside the solenoid coil of a rf probe capacitively tuned and matched to the 13C resonance frequency for B0 = 0.0487 T. Whole-body MRS acquisitions with flip angle (FA) = 18.2° every 2 s were started at the beginning of HCA production. The decay time constant for HP 13C-SUX post-injection was ~35 s (not accounting for rf-pulse associated losses). The well-resolved 13C spectrum of HP 13C-SUX shows a complex 13C multiplet: a result of spin-spin couplings between the hyperpolarized 13C isotopic label and 1H and 2H sites of HP 13C-SUX, Figure 1c.7 Moreover, the satellite peaks of the 13C multiplet (also due to spin-spin couplings between 13C and 1H and 2H nuclei) are also well resolved in vivo, Figure 3, inset, because the corresponding peak splittings are clearly visible when compared to the phantom MR spectrum, Figure 1c.
The use of HP 13C-SUX was demonstrated for low-field in vivo MR paving the way for future low-field MRS and MRI of 13C HCAs.
Acknowledgements
We appreciate support by NIH 1R21EB018014, 1R21EB020323, 1F32EB021840, T32 EB001628, and R01 CA160700 (W.P.) and NSF CHE- 1416268, DOD CDMRP W81XWH-12-1-0159/BC112431, and W81XWH-15-1-0271.References
[1] Coffey, A.M., Truong, M.L. & Chekmenev, E.Y. Low-field MRI can be more sensitive than high-field MRI. J Magn Reson 237, 169-174 (2013).
[2] Suefke, M., Liebisch, A., Blümich, B. & Appelt, S. External high-quality-factor resonator tunes up nuclear magnetic resonance. Nat Phys 11, 767-771 (2015).
[3] Sjolander, T.F., Tayler, M.C.D., King, J.P., Budker, D. & Pines, A. Transition-Selective Pulses in Zero-Field Nuclear Magnetic Resonance. J Phys Chem A 120, 4343-4348 (2016).
[4] Zacharias, N.M., Chan, H.R., Sailasuta, N., Ross, B.D. & Bhattacharya, P. Real-time molecular imaging of tricarboxylic acid cycle metabolism in vivo by hyperpolarized 1-(13)C diethyl succinate. J Am Chem Soc 134, 934-943 (2012).
[5] Bhattacharya, P., Chekmenev, E.Y., Perman, W.H., Harris, K.C., Lin, A.P., Norton, V.A., Tan, C.T., Ross, B.D. & Weitekamp, D.P. Towards hyperpolarized (13)C-succinate imaging of brain cancer. J Magn Reson 186, 150-155 (2007).
[6] Coffey, A.M., Shchepin, R.V., Truong, M.L., Wilkens, K., Pham, W. & Chekmenev, E.Y. Open-Source Automated Parahydrogen Hyperpolarizer for Molecular Imaging Using 13C Metabolic Contrast Agents. Anal. Chem. 88, 8279-8288 (2016).
[7] Chekmenev, E.Y., Hovener, J., Norton, V.A., Harris, K., Batchelder, L.S., Bhattacharya, P., Ross, B.D. & Weitekamp, D.P. PASADENA hyperpolarization of succinic acid for MRI and NMR spectroscopy. J Am Chem Soc 130, 4212-4213 (2008).
Figures