3038
Examination of the hyperpolarizability of fluorinated nicotinic acids and further pyridine carboxylic acids using SABRE1Department for Biometrics and Medical Informatics, Otto-von-Guericke University Magdeburg, Magdeburg, Germany, 2Eduard-Zintl-Institute for Inorganic and Physical Chemistry, Technical University Darmstadt, Darmstadt, Germany, 3Institute of Physical and Theoretical Chemistry, University of Bonn, Bonn, Germany
Synopsis
Nicotinic acid and isonicotinic acid are two derivatives of pyridine carboxylic acids. Both substrates are of high interest in medical chemistry. In the current study seven fluorinated derivatives of these pyridine carboxylic acids were chosen for examination of the 1H and 19F hyperpolarizability using the SABRE technique. Influences of the position of the carboxylic group and fluorine as well as the catalyst system concerning the achievable signal enhancements were examined. Furthermore an H/D-exchange could be observed in some cases. The presented data gives important information for future MR imaging studies.
Introduction
Nicotinic acid and isonicotinic acid are two derivatives of pyridine carboxylic acids. Both substrates are of high interest in medical chemistry. Nialamide, iproniazid, isoniazid and ethionamide are antidepressants or antibiotics and their molecular structure based on isonicotinic acid. Furthermore, numerous biochemistry studies have been done in presence of nicotinic acid. Here we choose seven fluorinated derivatives of these two pyridine carboxylic acids for examination the 1H and 19F hyperpolarizability using the Signal Amplification By Reversible Exchange (SABRE) technique.1-2 In SABRE experiments the symmetry break, which converts the parahydrogen spin order into polarization, is caused by the simultaneous addition of para-H2 and the fluorinated substrate to an Ir-center of the catalyst. A repeated hyperpolarization of the free substrate in solution is possible because of a continuous ligand exchange. As with the SABRE approach, the hyperpolarization generated in a PHIP experiment is not solely limited to the added protons but can be transferred to other MR-active spins (such as other protons or heteronuclei).Methods
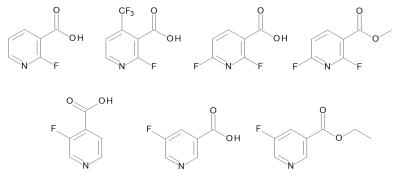
2-Fluor-3-pyridinecarboxylic acid, 2,6-difluoronicotinic acid, 3-fluoro-4-pyridinecarboxylic acid, 5-fluoro-3-pyridinecarboxylic acid, 2-fluoro-4-(trifluoromethyl)pyridine-3-carboxylic acid, 2,6-difluoro-3-pyridinecarboxylic acid methyl ester respectively 5-fluoro-nicotinic acid ethyl ester (see figure 1) were dissolved in 2 ml CD3OD in 10 mm NMR tube. After addition of the corresponding Ir catalyst (a) IMes-catalyst, b) Crabtree’s catalyst), the sample was degassed by using argon and ultrasonic bath for 5 minutes. The hyperpolarization was realized with about 50% enriched parahydrogen and 6 bar pressure. Directly after hydrogenation at about 6 mT, a 1H NMR spectrum was detected by using a single pulse experiment with a 90°excitation pulse on a Bruker wide bore 300 MHz spectrometer. Additionally, 19F NMR spectra were measured under same reaction conditions. The obtained signal enhancements (SEs) were calculated from signal-to-noise ratios of the thermal and the hyperpolarized spectra.Results and Discussion
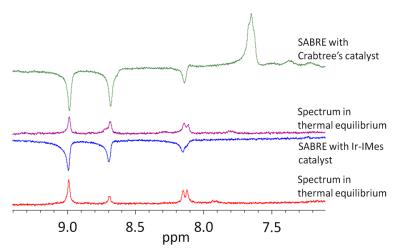
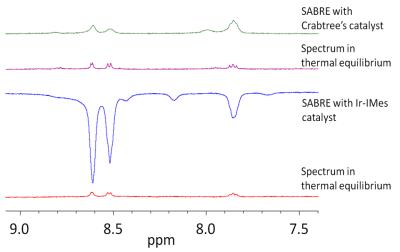
While no hyperpolarization or only very small polarized signals were observed for the ortho-fluorinated pyridine carboxylic acids, clear signal enhancements are detectable for all other examined substrates. For example, the 1H NMR spectra of 3-fluoro-4-pyridinecarboxylic acid and 5-fluoro-3-pyridinecarboxylic acid are shown in figure 2 and 3. The best signal enhancement is observable in presence of the Ir-IMes catalyst. Enhancements of more than 20 were calculated for the 1H signals.
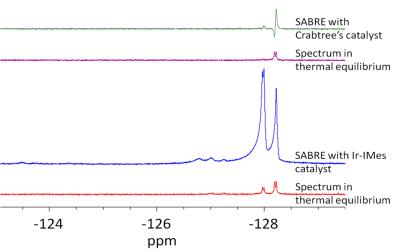
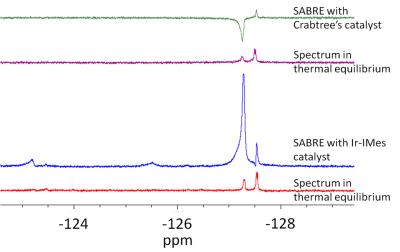
Additionally, 19F NMR measurements in CD3OD show maximal signal enhancements of about 10. As shown in figure 4 and 5 more than one fluorine signal is observable. Especially in case of the Ir-IMes catalyst, an H/D-exchange of some pyridine protons was detected. This leads to a new coupling system and influenced the polarization transfer.
Furthermore, 19F NMR signals of coordinated ligands are also detectable. These signals are also increase in the “SABRE-spectrum”.
Conclusion
Less is known about the hyperpolarization of 19F. Until now, only the polarization of fluoro pyridine (using SABRE) is published. In the current study, the first fluorinated pyridine carboxylic acids were hyperpolarized. Influences of the position of the carboxylic group and the catalyst system concerning signal enhancements and H/D-exchange could be observed in the presented NMR spectra. These results give new important information for future studies in the field of hyperpolarization using the SABRE technique.Acknowledgements
This work was supported by the Deutsche Forschungsgemeinschaft (BE 1824/12-1).References
1. Adams RW, et al. Reversible Interactions with para-Hydrogen Enhance NMR Sensitivity by Polarization Transfer. Science, 2009; 323: 1708-1711.
2. Pravdivtsev AN, et al. Transfer of SABRE-derived hyperpolarization to spin-1/2 heteronuclei. RSC Adv., 2015; 5: 63615-63623.
Figures