3037
Simultaneous Visualization of Hyperpolarized Fluorinated Amino Acids by Multi Chemical Shift Selective 19F MRI1Experimental Cardiovascular Imaging, Heinrich Heine University, Düsseldorf, Germany, 2Molecular Cardiology, Heinrich Heine University, Düsseldorf, Germany
Synopsis
We demonstrate the induction of 19F photo-chemically induced dynamic nuclear polarization (photo-CIDNP) in 19F MR imaging experiments. To this end, we made use of laser-induced hyperpolarization in a system consisting of flavin mononucleotide as a photosensitizer and the fluorinated aromatic amino acids tyrosine and tryptophan, respectively. The induction of 19F photo-CIDNP led to an extensive 19F signal enhancement which could be exploited for simultaneous imaging of both amino acids by 19F multi chemical shift-selective imaging within 20 s. Hence, our approach resulted in a substantial improvement of the intrinsically low 19F MR sensitivity for mono-fluorinated amino acids.
Introduction
Fluorinated analogues of amino acids have gained widespread attention as building blocks that may endow peptides and proteins with advantageous biophysical, chemical and biological properties. The in situ tracking of these compounds by 19F MR techniques is yet hampered by their rather low fluorine load in comparison to perfluorocarbons. To overcome this restriction, 19F photo-chemically induced dynamic nuclear polarization (photo-CIDNP) has been used to enhance their MR sensitivity and employed for mechanistic studies of photochemical reactions,1-3 for the determination of protein structures in their native and denatured states,4, 5 as well as for probing protein-ligand interactions.6 However, up to now the application of 19F photo-CIDNP was confined to MRS experiments at very high fields. The aim of the present study was to extend its use also for 19F MRI experiments. To this end, we made use of a 19F photo-CIDNP system consisting of flavin mononucleotide (FMN) as a photosensitizer and the fluorinated aromatic amino acids tyrosine and tryptophan, respectively (Figure 1), and performed multi chemical shift-selective imaging (mCSSI) for simultaneuos imaging of both amino acids.Methods
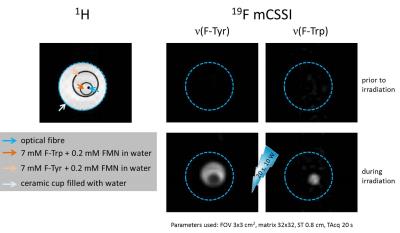
MR experiments were performed at 9.4 T using a 10-mm 1H/19F probe (for high sensitive, non-volume selective spectroscopy) or an actively shielded 57 mm gradient set in combination with a 30-mm 1H/19F birdcage resonator. For the spectroscopic 19F photo-CIDNP investigations, individual mixtures of 3-F-DL-tyrosine (F-Tyr, 4 mM) and FMN (0.2 mM) or of 6-F-DL-tryptophan (F-Trp, 4 mM) and FMN (0.2 mM) were prepared in D2O. The samples for imaging purposes were slightly higher concentrated with 7 mM F-Tyr and 0.2 mM FMN as well as 7 mM F-Trp and 0.2 mM FMN in water. For induction of photo-CIDNP, an air-cooled laser diode was coupled to an optical fibre equipped with a diffusor tip for uniform scattering of the light within the sample. The samples were irradiated with a wavelength of 450 nm and a power of 10 W either for 10 s (MRS) or 20 s (MRI). In the initial spectroscopy experiments, the CIDNP effect was monitored in individual experiments for tyrosine and tryptophan, respectively. For imaging experiments, both samples were nested in differently sized glass NMR tubes, which allowed their concurrent exposition to the laser pulse (Figure 2). We used an in-house developed multi chemical shift-selective imaging (mCSSI) sequence7 to simultaneously observe the different 19F resonance frequencies arising from F-Tyr and F-Trp within the same experiment in an acquisition time of 20 s.Results
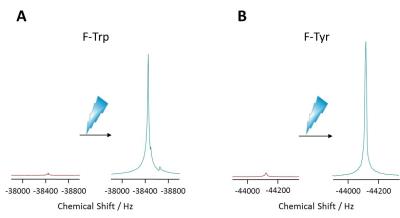
Figure 3 shows representative proton-decoupled 19F MR spectra of individual F-Tyr and F-Trp samples (4 mM each, 1 scan) acquired before and after induction of photo-CIDNP. As can be recognized, the 10 W laser pulse at 450 nm resulted in a tremendous increase in the 19F MR signal for both amino acids. Quantification of these data revealed a maximum signal enhancement of factor 60 for F-Tyr and 80 for F-Trp, respectively. While the signal amplification of F-Tyr was observed to be stable over several experiments, the extent of the photo-CIDNP effect for F-Trp already decreased after 4 laser cycles. Since the 19F MR resonances for both amino acids were found to be well resolved by a distance of ~5700 Hz (Figure 3), we aimed in the next step to image both compounds simultaneously by multi chemical shift-selective imaging. For an unequivocal signal assignment, both samples were spatially separated (Figure 2) and imaged with the same acquisition parameters before and after initiation of the laser pulse. While under basal conditions the 20 s acquisition led to noisy 19F MR images only, induction of 19F photo-CIDNP resulted in a clear visualization of both F-Tyr and F-Trp at the respective excitation frequencies (Figure 4; SNR: F-Tyr 40.6, F-Trp 5.9). Interestingly, the signal arising from the 19F nuclei in tryptophan led to a lower signal intensity compared to the 19F signal originating from F-tyrosine, which is somewhat contradictory to the initial spectroscopic observation described above. Clearly, further studies are required to resolve this issue.Conclusion
Our results show a strong 19F SNR enhancement by photo-CIDNP and, hence, a substantial improvement of the intrinsically insensitive 19F MR imaging technique for mono-fluorinated amino acids in the low millimolar range. Our first attempt to transfer 19F photo-CIDNP from spectroscopy to imaging opens up new pathways for basic research and holds the potential to give further insight into mechanisms of enzymatic reactions, where alterations in the 19F chemical shift might be exploited for an in situ visualization of molecular transformations by a combination of 19F photo-CIDNP and mCSSI.Acknowledgements
The authors would like to thank Thomas Walter (Walter Laser, München) for the custom-made laser diode.References
(1) Feldmeier C, Bartling H, Riedle E, Gschwind RM. LED based NMR illumination device for mechanistic studies on photochemical reactions--versatile and simple, yet surprisingly powerful. J Magn Reson 2013; 232:39-44.
(2) Feldmeier C, Bartling H, Magerl K, Gschwind RM. LED-illuminated NMR studies of flavin-catalyzed photooxidations reveal solvent control of the electron-transfer mechanism. Angew Chem Int Ed Engl 2015; 54(4):1347-51.
(3) Wolff C, Kind J, Schenderlein H et al. Studies of a photochromic model system using NMR with ex-situ and in-situ irradiation devices. Magn Reson Chem 2016; 54(6):485-91.
(4) Khan F, Kuprov I, Craggs TD, Hore PJ, Jackson SE. 19F NMR studies of the native and denatured states of green fluorescent protein. J Am Chem Soc 2006; 128(33):10729-37.
(5) Mok KH, Kuhn LT, Goez M et al. A pre-existing hydrophobic collapse in the unfolded state of an ultrafast folding protein. Nature 2007; 447(7140):106-9.
(6) Lee Y, Zeng H, Ruedisser S, Gossert AD, Hilty C. Nuclear magnetic resonance of hyperpolarized fluorine for characterization of protein-ligand interactions. J Am Chem Soc 2012; 134(42):17448-51.
(7) Jacoby C, Oerther T, Temme S, Schrader J, Flögel U. Simultaneous 19F MR imaging at different resonance frequencies using multi chemical shift selective RARE. ISMRM 2014, Milan; 2927.
Figures