3036
photo-CIDNP for 19F MR amino acid-protein interaction studies in physiological solvents1Department for Biometrics and Medical Informatics, Otto-von-Guericke University Magdeburg, Magdeburg, Germany, 2Institute of Physical and Theoretical Chemistry, University of Bonn, Bonn, Germany
Synopsis
Fluorinated amino acids are of high interest in biochemistry and pharmaceutics. The low 19F MR signal was increased employing hyperpolarization (photo-Chemical Induced Dynamic Nuclear Polarization, photo-CIDNP). 3-Fluoro-tyrosine in physiologic salt solution was hyperpolarized in presence of an albumin derivative using a low cost LED allowing repetitive irradiation and increasing the hyperpolarized signal. A clear 19F signal enhancement could be observed. In comparison to past examinations with a laser system, only small temperature changes caused by LED light irradiation were measured in the current study.
Introduction
Fluorinated amino acids are of high interest in biochemistry and in pharmaceutics.1 MR techniques are well suited to examination drug metabolism or even localizing the substrate. 19F with its lacking natural abundance in living organisms and high chemical shift sensitivity due to changes in its surrounding (e.g. temperature or pH value) is a promising molecular probe. But the 19F signal is usually much weaker than the 1H signal rendering 19F NMR or 19F MRI difficult. To increase the sensitivity of heteronuclei, hyperpolarization techniques such as photo-Chemical Induced Dynamic Nuclear Polarization (photo-CIDNP) can be used.2-3 We present an MR spectroscopic study of hyperpolarizing 3-fluoro-tyrosine in physiologic salt solution in the presence of a protein using a low cost LED set-up.4-5Methods
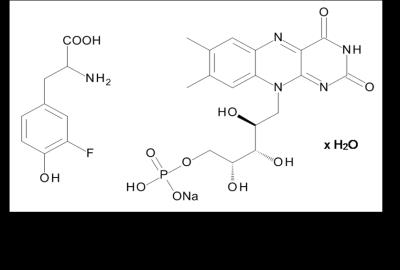
3 mg 3-Fluoro-DL-tyrosine and 3 mg riboflavin 5’-monophosphate sodium salt hydrate (see figure 1) were dissolved in 2 ml physiologic salt solution in a 10 mm NMR tube. A 5 mm NMR tube, filled with 600 µl D2O was inserted as a reference substrate into the 10 mm NMR tube. In a first examination an optical fibre connected to a Cree XP E high power LED (455 nm) was centrally positioned in the D2O solution. For comparison, this LED was also positioned directly in the physiologic salt solution. The LED-device (3.4 V, ~ 400 mA) was controlled by a pulse program of a 7 T NMR spectrometer (Bruker WB-300 Ultrashield) allowing complete automatic control of irradiation from within the sequence including repetitive irradiation. Irradiation times were between 0 s and 15 s. To study the interaction between 3-fluoro-DL-tyrosine and protein (bovine albumin), measurements before and after addition of 50mg bovine albumin to the solution, were performed. 1H MR spectra were detected using a 45° pulse and 90° pulse respectively (P1=14.5 µs / 29 µs, PL1 = 17 W). 19F NMR spectra were measured using a 90° pulse (P1 = 32.5 µs, PL1 = 17 W).Results and Discussion
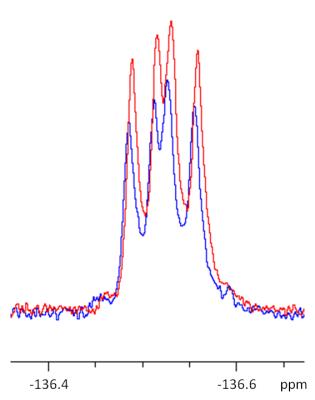
In the chemical shift region of 3-fluoro-DL-tyrosine, no hyperpolarized 1H signals could be observed. In contrast to these findings, a clear 19F signal enhancement could be observed for the chosen system subsequently after irradiation. The figures 2 and 3 show exemplary signals after 0 s and 12 s irradiation time at 300K. Only small temperature changes (+ 0.1 K) caused by LED light irradiation could be measured in all examinations.
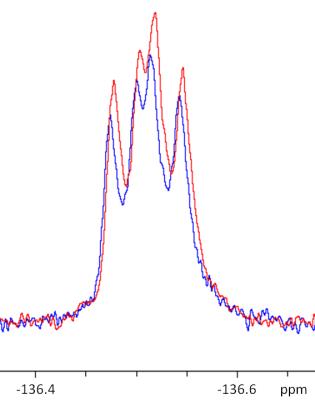
In comparison to the measurements shown above, experiments in smaller volumes (5 mm NMR tube with 600 µl solvent) leads to higher signal enhancements as shown in figure 4.
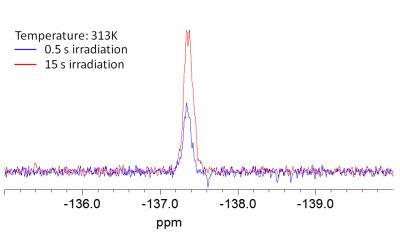
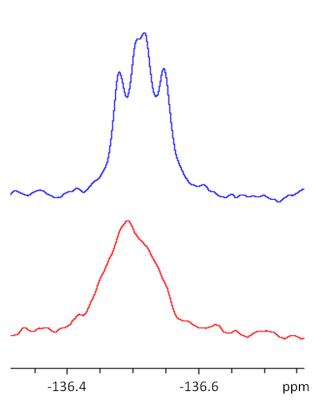
The molecular interaction between 3-fluoro-DL-tyrosine and bovine albumin can be detected as a chemical shift change of the 19F MR signal towards lower field strengths, as well as broadening of the 19F MR signal, as shown in figure 5.
Conclusion
Previous studies of the system 3-fluoro-DL-tyrosine and riboflavin 5’-monophosphate sodium salt hydrate by our group were performed at a different temperature and using D2O or physiologic salt solution as solvent.6 Now, the new results show that 19F enhancements could also be detected in presence of bovine albumin which may help to disentangle interactions between proteins and amino-acids.Acknowledgements
No acknowledgement found.References
1. Ojima I, Fluorine in Medical Chemistry and Chemical Biology, 1.Ed., Wiley-Blackwell: Chichester, 2009.
2. Bargon J, et al. Kernresonanz-Emissionslinien während rascher Radikalreaktionen. Zeitschrift Naturforschung Teil A, 1967;22:1551-1555.
3. Goez M. Photo-CIDNP spectroscopy. Annual reports on NMR Spectroscopy, 2009;66:77-147.
4. Feldmeier C, et al. LED based NMR illumination device for mechanistic studies on photochemical reactions – Versatile and simple, yet surprisingly powerful. J. Magn. Res., 2013;232:39-44.
5. Feldmeier C, et al. LED-Illuminated NMR Studies of Flavin-Catalyzed Photooxidations Reveal Solvent Control of the Electron-Transfer Mechanism. Angew. Chem., 2015;127:1363-1367.
6. Euchner F, et al. LED induced 19F hyperpolarization in physiological solvent. MAGMA. 2016;29(1):S148-S149.
Figures