3032
Hyperpolarization of 2-keto[1-13C]isocaproate for in vivo studies with photo-induced radicals1Laboratory for Functional and Metabolic Imaging, École Polytechnique Fédérale de Lausanne, Lausanne, Switzerland, 2Institute of Physics of Biological Systems, Swiss Federal Institute of Technology, Lausanne, Switzerland, 3General Electric Healthcare, Chalfont Saint Giles, United Kingdom, 4Department of Radiology, University of Lausanne, Lausanne, Switzerland, 5Department of Radiology, University of Geneva, Geneva, 6Centre for Biomedical Imaging, École Polytechnique Fédérale de Lausanne, Lausanne, Switzerland
Synopsis
Hyperpolarized 2-keto[1-13C]isocaproate (KIC) provides a means to probe brain nitrogen homeostasis and to assess molecular signatures of tumors. The dynamic nuclear polarization process requires a free-radical polarizing agent, and samples are typically doped with persistent radicals. An alternative is to use photo-induced radicals of α-keto acids that recombine upon dissolution. [1-13C]KIC hyperpolarized with photo-induced radicals could be used to measure the alterations in amino acid metabolism that are linked to neurodegenerative diseases and cancer, and the aim of the present study is to identify the main features that influence the polarization dynamics.
Introduction
Branched-chain aminotransferase (BCAT) plays an important role in the astroglial glutamate/glutamine shuttle, and it provides a buffering mechanism for adapting glutamate concentrations [1]. It has been demonstrated that, upon injection of hyperpolarized 2-keto[1-13C]isocaproate ([1-13C]KIC), BCAT activity can be assessed in the rodent brain by measuring its metabolic product [1-13C]leucine. This provides a way to probe brain nitrogen homeostasis [2] and to assess molecular signatures of tumors [3]. For dynamic nuclear polarization (DNP) a polarizing agent exhibiting a free electron (radical), is needed to transfer the much higher spin polarization of electrons to nuclei by microwave irradiation [4]. The persistent radicals typically employed accelerate the relaxation of the signal upon dissolution and must be removed prior to injection for clinical applications. Transient radicals generated by photo-excitation of frozen α-keto acids represent a promising alternative. Eichhorn et al. [5] have demonstrated the feasibility of photo-induced radicals of pyruvic acid (PA) for DNP. The photo-induced radicals have the suitable property of being stable at low temperatures (liquid nitrogen) and recombine to non-radical species upon dissolution [5]. The aim of the present study is to show that it is feasible to generate radicals in KIC through the same mechanism for hyperpolarized 13C MRS and to characterize the polarization dynamics.Methods
Droplets of neat [1-13C]KIC (free acid, 8.3 M, Sigma Aldrich) or unenriched KIC mixed with toluene or tert-butanol were dispersed one by one into a quartz dewar filled with liquid nitrogen. The radicals were produced within the frozen droplets at 77 K upon irradiation with UV light (365 nm, Hamamatsu UV-LED). The overall radical concentration of the UV-irradiated KIC droplets were measured at 77 K by EPR, with calibration using TEMPOL radical. To study the spatial radical distribution via EPR, UV-irradiated KIC droplets and KIC in capillary tubes with varying cross section area were examined. The frozen UV-irradiated [1-13C]KIC samples were polarized in a 7 T custom-designed polarizer (196.8 GHz / 1 K). The solid-state build-up of the polarization was monitored with low flip angle pulses. The NMR signal enhancement was determined after rapid dissolution and transfer (3s) to a home-built separator-infusion pump placed in a 9.4 T/ 31 cm animal scanner (Varian/Magnex). The hyperpolarized signal decay was monitored with a series of pulse-acquire scans (5° flip angle, 3s repetition time) using a home-built single-loop coil wound around the separator-infusion pump [6]. The same 5° pulse was used to measure the thermal signal.Results and Discussion
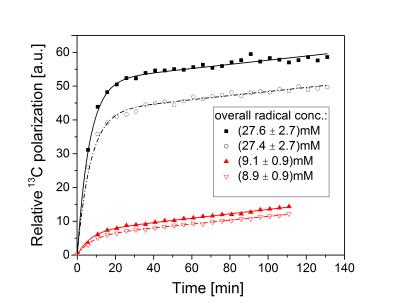
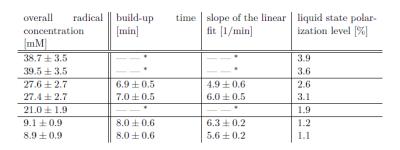
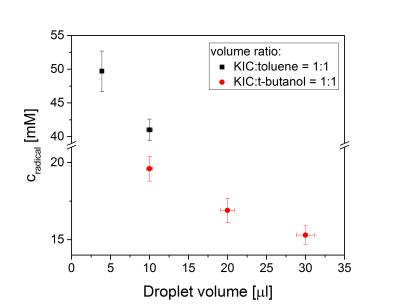
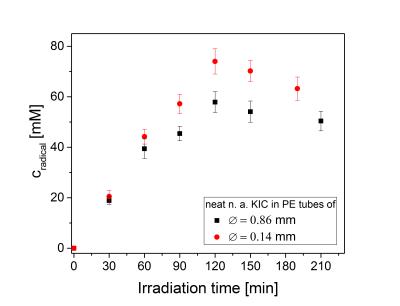
The polarization build-up of [1-13C]KIC with UV-induced radicals is strikingly different from that seen using stable persistent radicals (e.g., trityl [7] ,and nitroxyl [8]). Instead of a mono-exponential build-up, the [1-13C]KIC samples polarize rapidly at the beginning and then continue to polarize slowly (see Fig. 1). The build-up curve was modeled with a two component system, in which the component exhibiting a slow exponential build-up was approximated with a linear fit function. The liquid-state polarization level rises with increasing overall radical concentration, whereas the polarization dynamics remain approximately constant (see Table 1). These results suggest that the spatial distribution of the radical is inhomogeneous within the UV-irradiated frozen KIC droplets. EPR experiments with UV-irradiated droplets of diluted KIC or KIC in capillary tubes of varying volume show that the overall radical concentration decreases in samples with a larger cross section area (see Fig. 2 and 3). This seems to remain valid independently of the illumination time and the solvent. Interestingly, UV-irradiated [1-13C]PA prepared under the same conditions does not show a slow exponential component of the polarization build-up at 1.2 K, 5 T [5]. The results show that the radical distribution strongly affects the polarization dynamics. The evidence of a spatially inhomogeneous radical distribution suggests that the UV-light is mostly absorbed at the surface of the droplets and the radical formation is limited to this region.Conclusions
Since the average radical concentrations are high, UV-irradiated KIC is a promising candidate to perform hyperpolarized in vivo studies. This study reveals an issue that has to be overcome to make photo-generated radicals in α-keto acids competitive to persistent radicals. Future work will focus on modified irradiation conditions to achieve a more spatially homogeneous radical distribution and a higher overall polarization level.Acknowledgements
This work was supported by the Swiss State Secretariat for Education, Research and Innovation (SERI) within the Marie Curie Initial Training Network EUROPOL project (n° SERI: 15.0164).References
[1] M. Yudkoff, et al., Astrocyte leucine metabolism: significance of branched chain amino acid transamination, J. Neurochem. 66 (1) (1996) 378-385
[2] S. A. Butt, et al., Imaging cerebral 2-ketoisocaproate metabolism with hyperpolarized 13C magnetic resonance spectroscopic imaging, Blood Flow. Metab. 32 (8) (2012) 1508-1514
[3] M. Karlsson et al., Imaging of branched chain amino acid metabolism in tumors with hyperpolarized 13C ketoisocaproate, International Journal of Cancer 127 (3) (2010) 729-736
[4] W. T. Wenckebach, The Solid Effect, Appl. Mag. Reson. 34 (2008) 227-235
[5] T. R. Eichhorn et al., Hyperpolarization without persistent radicals for in vivo real-time metabolic imaging, Proc. Natl. Acad. Sci. U.S.A. 110 (45) (2013) 18064–18069
[6] T. Cheng et al., Automated transfer and injection of hyperpolarized molecules with polarization measurement prior to in vivo NMR, NMR in biomedicine 26 (11) (2013) 1582–1588
[7] J. H. Ardenkjaer-Larsen, et al., Increase in signal-to-noise ratio of > 10,000 times in liquid-state NMR, Proc. Natl. Acad. Sci. U.S.A. 100 (18) (2003) 10158-10163
[8] T. Cheng et al., Over 35% liquid-state 13C polarization via dissolution dynamic nuclear polarization at 7 T and 1 K with ubiquitous nitroxyl radicals, Phys. Chem. Chem. Phys. 15 (2013) 20819-20822
Figures