3024
MEGA-PRIAM: Dual-volume excitation and parallel reconstruction for J-difference-edited MR spectroscopy1Russell H. Morgan Department of Radiology and Radiological Science, The Johns Hopkins University School of Medicine, Baltimore, MD, United States, 2F.M. Kirby Research Center for Functional Brain Imaging, Kennedy Krieger Institute, Baltimore, MD, United States, 3Department of Biomedical Engineering, The Johns Hopkins University School of Medicine, Baltimore, MD, United States, 4Hvidovre Hospital, Danish Research Center for Magnetic Resonance, Hvidovre, Denmark
Synopsis
A twofold acceleration of MEGA-edited spectroscopy, using Parallel Reconstruction In Accelerated Multivoxel (MEGA-PRIAM) to simultaneously acquire data in two separate locations, is demonstrated. PRIAM separates the signals from two dualband-excited voxels using spatial receiver-coil sensitivity profiles. Phantom experiments show that MEGA-PRIAM separates GABA- and glutathione-edited spectra with low crosstalk between the voxels. In-vivo experiments establish that dual-voxel MEGA-PRIAM increases signal-to-noise ratio (SNR) 40% compared to sequentially acquired single-voxel MEGA-PRESS measurements with the same total duration. GABA and glutathione estimates are consistent between dual-voxel MEGA-PRIAM and single-voxel MEGA-PRESS acquisitions.
Purpose
Edited MRS measurements of low-concentration metabolites such as GABA 1 and glutathione (GSH) 2 typically require acquisition times on the order of 10 minutes due to low signal-to-noise ratio (SNR). Study designs are therefore limited in the number of brain regions that can be investigated within a research protocol. Simultaneous and separable acquisition of MR spectra from two voxels can be achieved through dual-voxel excitation in combination with Parallel Reconstruction In Accelerated Multivoxel (PRIAM 3), based on multi-channel coil sensitivity encoding. Here, PRIAM and MEGA (MEGA-PRIAM) are combined for dual-voxel editing at 3T. Phantom and in-vivo experiments establish that MEGA-PRIAM effectively doubles the data acquisition rate of edited MRS.Methods
PRIAM uses a parallel imaging SENSE-type 4 reconstruction to separate the MRS time-domain signals from two simultaneously excited voxels. A gradient-echo acquisition establishes a complex receiver-coil sensitivity map, normalizing the signal from each receiver channel to the sum-of-squares. A complex sensitivity matrix is calculated by averaging these maps across each spectroscopic voxel. The SENSE unfolding matrix is calculated from this sensitivity matrix and the noise correlation matrix. Applying this matrix to the time-domain signals from all receiver channels gives optimal separation of the MRS signals from the two spectroscopic voxels.
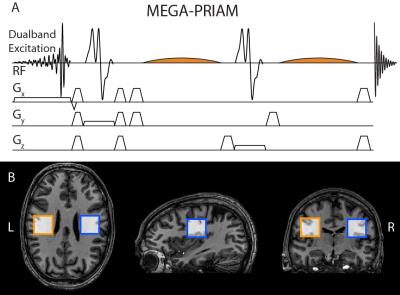
Dual-band excitation was achieved by point-by-point summation of two slice-selective excitation pulses, implemented in the MEGA-PRESS pulse sequence (Figure 1A).
Parameters for J-difference editing of GABA/GSH: TE = 68/120 ms, 27/36 ml voxel size, 12/20 ms editing pulse, ON pulse at 1.9/4.56 ppm, OFF pulse at 7.46/8.00 ppm; TR = 2 s, 3T Philips Achieva, 32-channel phased-array volume head coil.
Phantom: A two-compartment agarose phantom was prepared (first compartment: 16 mM GABA, 10 mM GSH; second compartment: 8 mM GABA, 14 mM GSH). Dual-voxel MEGA-PRIAM was performed with one voxel in each compartment (and single-voxel MEGA-PRESS for each) for 8 averages. Signal cross-talk was calculated from a single unsuppressed water reference scan.
In-vivo: 11 healthy adults gave written informed consent with local IRB approval. Data were acquired from two voxels placed symmetrically about the midline in the left and right posterior frontal lobes (Figure 1B), using the same parameters as for the phantom data, except with 320 (GABA) and 400 (GSH) averages for dual-voxel acquisition, and 160 (GABA) and 200 (GSH) averages for each single-voxel acquisition, i.e. with same total scan duration. Post-processing, averaging, and fitting was performed with Gannet 2.0 5 including frequency and phase correction (Spectral Registration 6 for GABA, NAA peak referencing for GSH). Metabolite levels and SNRs were calculated. To estimate the reduction in SNR resulting from very similar coil sensitivity profiles for both spectroscopic voxels, g-factors were calculated according to the SENSE formalism.3,4
Results
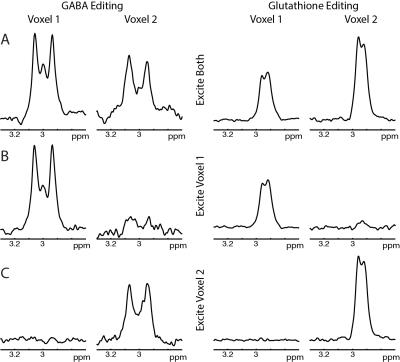
Phantom: GABA- and GSH-edited spectra from dual-voxel excitation show good agreement with single-voxel spectra (shown in Figure 2). Average crosstalk was ~4.0% for both GABA and GSH experiments. The GABA concentration ratio (expected: 2.00) between the two voxels was 2.04 (dual-voxel) and 2.03 (single-voxel). The GSH concentration ratio (expected: 0.71) was 0.76 (dual-voxel) and 0.85 (single-voxel).
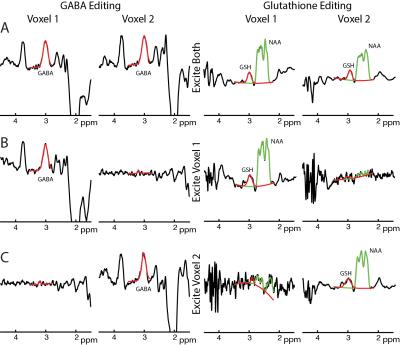
In-vivo: Reconstructed GABA- and GSH-edited dual-voxel spectra show good agreement with sequentially acquired single-voxel spectra (Figure 3). Dual-voxel acquisitions (both voxels for full duration) showed a mean SNR increase of 1.38±0.24 compared to the single-voxel experiments (each voxel for half duration), close to the theoretical √2 improvement. There was no significant difference between dual-voxel and single-voxel estimates of GABA or GSH. On average, GABA level estimates between dual-voxel and single-voxel measurements agreed to within 2% (7% for GSH), demonstrating that the dual-voxel approach does not bias concentration estimates. Mean g-factors were 1.025±0.017 for GABA editing (GSH: 1.026±0.011), suggesting excellent signal separability with low mutual noise amplification.
Discussion
Phantom and in-vivo data show good agreement between simultaneously acquired dual-voxel MEGA-PRIAM and sequential single-voxel MEGA-PRESS spectra of the same total duration, with low signal crosstalk between voxels, increased SNR, and consistent metabolite concentration estimates. Effectively, MEGA-PRIAM accelerates the data acquisition rate of edited MRS by a factor of 2. The gain in temporal SNR will partly depend on the degree of voxel separation (as measured by the g-factor), and may require advanced shimming procedures.
MEGA-PRIAM can be extended to more than one voxel, and implemented within the recently demonstrated HERMES scheme 7,8 to allow simultaneous multi-voxel-multi-metabolite experiments with transformative potential for edited MRS studies.
Conclusion
MEGA-PRIAM allows the simultaneous acquisition of edited MRS measurements in half the scan time compared to sequential MEGA-PRESS acquisition while maintaining metabolite estimates and SNR.Acknowledgements
This work was supported by NIH grants R01 EB016089, and P41 EB015909.References
1. Puts
NAJ & Edden RAE. In vivo magnetic resonance spectroscopy of GABA:
A methodological review. Prog. Nucl. Magn. Reson. Spectrosc. 2012;60:29–41.
2. Terpstra M, Marjanska M, Henry PG, Tkác I & Gruetter R. Detection of an antioxidant profile in the human brain in vivo via double editing with MEGA-PRESS. Magn. Reson. Med. 2006;56:1192–1199.
3. Boer VO, Klomp DWJ, Laterra J & Barker PB. Parallel reconstruction in accelerated multivoxel MR spectroscopy. Magn. Reson. Med. 2015;74:599–606.
4. Pruessmann KP, Weiger M, Scheidegger MB & Boesiger P. SENSE: Sensitivity encoding for fast MRI. Magn. Reson. Med. 1999;42:952–962.
5. Edden RAE, Puts NAJ, Harris AD, Barker PB & Evans CJ. Gannet: A batch-processing tool for the quantitative analysis of gamma-aminobutyric acid–edited MR spectroscopy spectra. J. Magn. Reson. Imaging 2014;40:1445–1452.
6. Near J, Edden R, Evans CJ, Paquin R, Harris A & Jezzard P. Frequency and phase drift correction of magnetic resonance spectroscopy data by spectral registration in the time domain. Magn. Reson. Med. 2015;73:44–50.
7. Chan KL, Puts NAJ, Schär M, Barker PB & Edden RAE. HERMES: Hadamard encoding and reconstruction of MEGA-edited spectroscopy. Magn. Reson. Med. 2016;76:11–19.
8. Saleh MG, Oeltzschner G, Chan KL, Puts NAJ, Mikkelsen M, Schär M, Harris AD & Edden RAE. Simultaneous edited MRS of GABA and glutathione. NeuroImage 2016 (in press). doi:10.1016/j.neuroimage.2016.07.05
Figures