3023
Measuring and minimizing effects of eddy currents on selective spectral editing experiments at 3T1Russell H. Morgan Department of Radiology and Radiological Science, The Johns Hopkins University School of Medicine, Baltimore, MD, United States, 2F.M. Kirby Research Center for Functional Brain Imaging, Kennedy Krieger Institute, Baltimore, MD, United States, 3CAIR Program, Alberta Children's Hospital Research Institute, University of Calgary, Calgary, AB, Canada, 4Department of Radiology, University of Calgary, Calgary, AB, Canada, 5Hotchkiss Brain Institute and Alberta Children's Hospital Research Institute, Calgary, AB, Canada, 6Diagnostic Imaging and Radiology, Children’s National Health System, Washington, DC, United States, 7Department of Radiology, George Washington University, Washington, DC, United States, 8Department of Neurology, The Johns Hopkins University School of Medicine, Baltimore, MD, United States
Synopsis
Macromolecule-suppressed J-difference-edited MRS of GABA is extremely sensitive to B0 offsets. Relatively small frequency shifts (~10 Hz) may cause unwanted co-editing of macromolecules, to the extent that the edited ‘GABA’ signal appears negative in-vivo. We demonstrate an approach to measure transient field shifts arising from gradient-related eddy currents, and present a way to minimize these effects in order to restore correct editing.
Purpose
Symmetrical J-difference editing schemes increase the selectivity of editing by suppressing co-edited signals, e.g. macromolecular (MM) signals in GABA editing.1–3 However, highly selective spectral editing experiments are almost uniquely sensitive to frequency mis-calibration, as may occur due to B0 drift.4,5 Field gradient pulses (for selective dephasing, e.g. as ‘crusher’ gradients or in water suppression modules) are another potential source of frequency offset, as they induce eddy currents that cause transient B0 shifts. The purpose of this abstract is to demonstrate that gradient-related frequency offsets interfere with the symmetrical suppression of the MM signal in GABA-edited MRS.Methods
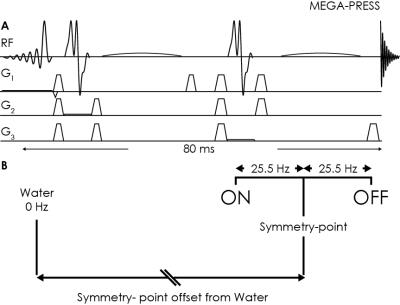
All experiments were performed on a Philips 3T Achieva scanner, using a MEGA-PRESS sequence (Figure 1A) with TR/TE = 2000/80 ms; 20-ms sinc-Gaussian editing pulses (bandwidth 53 Hz) at 1.9 and 1.5 ppm 2, symmetrically arranged about 1.7 ppm (the frequency of the coupled MM resonance) to suppress the co-edited MM signal. Two male subjects gave informed consent to participate.
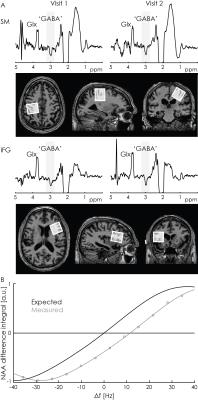
Initial in-vivo acquisition: MEGA-PRESS spectra were acquired twice from the inferior frontal gyrus and primary sensorimotor areas (27-mL voxels, 320 averages). Data were processed using the ‘Gannet’ program.6
Determination of eddy-current-induced frequency offsets: MEGA-PRESS saturation-offset series were acquired from an NAA phantom by varying the symmetry-point offset (in steps of 0.05 ppm). The intensity of the NAA signal at 2.01 ppm in the difference spectrum was measured. For correct frequency calibration at 3T, the zero-crossing of the NAA difference integral should appear when the symmetry-point has an offset of -353.7 Hz from the water frequency, corresponding to the difference between the chemical shifts of NAA and room-temperature water (Figure 1B). The deviation of the measured zero-crossing from this expected zero-crossing is defined as Δf, which quantifies transient changes in B0 at the time the editing pulse is applied - a correctly calibrated experiment has Δf=0.
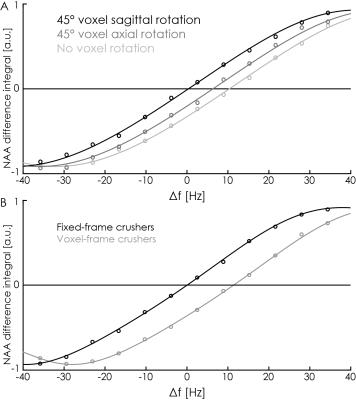
Voxel orientation effects: In order to investigate the impact of crusher-gradient orientation on editing pulse offsets, saturation-offset series (as above) were acquired for three different crusher gradient (voxel) orientations: no voxel rotation; 45-degree axial rotation; and 45-degree sagittal rotation.
Fixing the crusher-gradient coordinate-frame: Subsequently, the coordinate frame of crusher gradients was decoupled from the voxel orientation and fixed to a 45-degree sagittal rotation. Offset series (as above) were acquired from an unrotated voxel with and without this switch enabled.
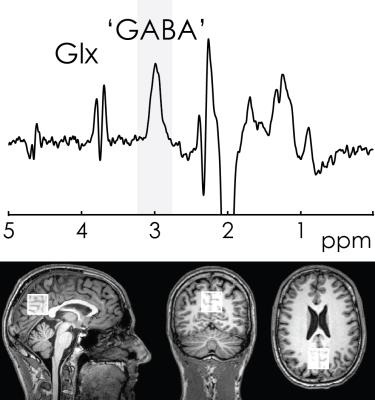
Final in-vivo acquisition: MEGA-PRESS data were acquired from a midline parietal region. Acquisition and post-processing were identical to the initial in-vivo acquisitions, except that the coordinate frame of crusher gradients was fixed.
Results
The initial in-vivo spectra showed a negative edited ‘GABA’ signal at 3 ppm (Figure 2A), due to negative co-edited MM signal, suggesting a substantial editing pulse frequency offset. This was supported by the phantom experiment (Δf = 11.5 Hz, Figure 2B). Δf was found to vary with voxel orientation: 10.4 Hz (unrotated); 6.4 Hz (45-degree axial rotation); 0.4 Hz (45-degree sagittal rotation) (Figure 3A). Fixing the coordinate frame of crusher gradients reduced Δf from 11.5 Hz to 0.1 Hz (Figure 3B). The in-vivo spectrum acquired with fixed crusher-gradient coordinate-frame showed the expected positive-edited ‘GABA’ signal (Figure 4).Discussion
MM-suppressed GABA-editing at 3T is very susceptible to frequency mis-calibration. Net-negative edited signals at 3 ppm may occur for editing pulse Δf >13 Hz (e.g. Fig. 3C in reference 5). The current experiments show a transient field shift of this order of magnitude during the editing pulses, the magnitude of which depends on crusher gradient orientation, unambiguously establishing that the effect is caused by gradient pulses. These eddy-current effects, while relatively small and transient, significantly affect the performance of highly selective spectral editing experiments. The effects can be minimized by fixing the crusher-gradient orientation to one with the least eddy current effects.
Magnitude-, duration- and orientation-dependence of eddy-current-related offset are likely to be heavily scanner-dependent. The transient frequency shift during editing pulses may be due to sub-optimal eddy-current compensation, as much as due to the eddy currents themselves.
Conclusion
For experiments with highly selective spectral-editing pulses, e.g. macromolecule-suppressed GABA editing or double-editing experiments 7 at 3T, it is crucial to test experimentally whether editing pulses are applied at the intended offsets. B0 eddy currents may result in incorrect editing pulse offsets and unwanted co-editing.Acknowledgements
This work was supported by NIH grants R01 EB016089, R01 MH106564, and P41 EB015909.References
1. Henry PG, Dautry C, Hantraye P, Bloch G. Brain GABA editing without macromolecule contamination. Magn. Reson. Med. 2001;45:517–520.
2. Edden RAE, Puts NAJ, Barker PB. Macromolecule-suppressed GABA-edited magnetic resonance spectroscopy at 3T. Magn. Reson. Med. 2012;68:657–661.
3. Harris AD, Puts NAJ, Barker PB, Edden RAE. Spectral-editing measurements of GABA in the human brain with and without macromolecule suppression. Magn. Reson. Med. 2014;74:1523–1529.
4. Harris AD, Glaubitz B, Near J, John Evans C, Puts NAJ, Schmidt-Wilcke T, Tegenthoff M, Barker PB, Edden RAE. Impact of frequency drift on gamma-aminobutyric acid-edited MR spectroscopy. Magn. Reson. Med. 2014;72:941–948.
5. Edden RAE,
Oeltzschner G, Harris AD, Puts NAJ, Chan KL, Boer VO, Schär M, Barker PB.
Prospective frequency correction for macromolecule-suppressed GABA editing at
3T. J. Magn. Reson. Imaging 2016 (in press).
6. Edden RAE, Puts NAJ, Harris AD, Barker PB, Evans CJ. Gannet: A batch-processing tool for the quantitative analysis of gamma-aminobutyric acid–edited MR spectroscopy spectra. J. Magn. Reson. Imaging 2014;40:1445–1452.
7. Terpstra M, Marjanska M, Henry P-G, Tkác I, Gruetter R. Detection of an antioxidant profile in the human brain in vivo via double editing with MEGA-PRESS. Magn. Reson. Med. 2006;56:1192–1199.
Figures