3021
In-vivo testing of automatic voxel prescription for high inter-subject reproducibility in single-voxel MR spectroscopy1Electrical Enginnering, Korea Advanced Institute of Science and Technology, Daejeon, Korea, Republic of, 2Department of Radiology, Center for Magnetic Resonance Research, University of Minnesota Medical School, Minneapolis, MN, United States, 3Department of Radiology, Duke University Medical Center, Durham, NC, United States, 4Department of Radiology, Johns Hopkins University School of Medicine, Baltimore, MD, United States
Synopsis
In this study, we present the implementation and outcome of a scheme for automatic voxel placement in single-voxel spectroscopy. The scheme is based on transfer of voxels prescribed on an atlas to the subject images during the scanning session and allows fast and reliable placement of voxels for spectroscopy measurements. 1H spectra of three different volumes of interest (VOIs) from multiple subjects were measured with a Siemens 3T scanner following automated and manual VOI placements. MRS data acquired using automatic placement produced spectral quality comparable to manual placement, while yielding better cross-subject spatial consistency than manual placement.
Purpose
During a typical MR Spectroscopy (MRS) study, the volume of interest (VOI) is manually selected by the MR technologist, which often induces some degree of variability. To rectify this issue, we have previously presented a scheme for automatic voxel placement in single-voxel spectroscopy using the registration of a brain atlas, where the VOI was prescribed by an expert, to the 3D T1-weighted subject’s data1. This registration-based method allows faster computation time than segmentation-based methods and can be utilized for any brain region pre-defined on an atlas rather than only for areas that can be segmented. The study also demonstrated that non-linear registrations improved voxel placement consistency across subjects over linear registrations by better accommodating anatomical variability. The goal in the current study was to compare spectral quality and spatial consistency obtained with atlas-based automated vs. manual VOI placement.Methods
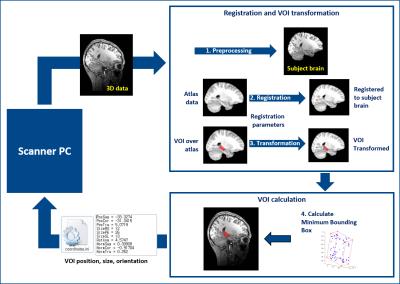
The automatic registration scheme uses 3D T1 volume data of the subject (Fig1). Once the scan is finished, the data are transmitted from scanner PC to another computation PC running the automatic placement code (AutoVOI) via ethernet. It initially generates registration parameters from atlas brain to the subject brain, then transforms the VOI defined over the atlas to the subject space using the registration parameters. Once the transformation is finished, it runs a brute force minimum bounding box algorithm2 to determine the position and orientation of the target VOI in the subject space, which then is transferred to the MRS localization sequence via a text file. In this test, T1 MPRAGE (TR/TI/TE=2530/1100/3.65ms, 1mm3 resolution) data were acquired using a 3T Siemens Verio scanner with a 32Ch receive array coil. A standard desktop PC running Linux was used for AutoVOI computation (Fedora23, i5-3570@3.40GHz, 8GB-RAM, NVIDIA-GT630@1GB-VRAM). AutoVOI code ran on MATLAB, while utilizing FSL-BET3 and 3D volume registration and transformation from BROCCOLI4 package.
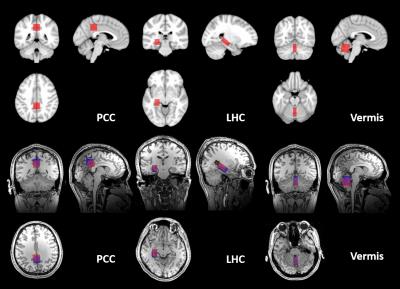
Spectra were acquired from posterior cingulate cortex (PCC) (20x20x20mm3), left hippocampus (LHC) (13x26x12mm3) and the cerebellar vermis (10x25x25mm3). The VOI were defined over MNI152 1mm3 brain atlas based on prescriptions in prior papers5,6,7 (Fig2).
Six subjects (1 female, 24±2 years) were scanned for LHC, and five subjects (all male, 22±2 years) were scanned for PCC and Vermis. AutoVOI placements were followed by manual placements of the same VOIs. To attenuate bias in the manual placement, the exact placement of AutoVOI was hidden and only displayed in projected form over sagittal, coronal and axial scout images taken at the isocenter position. B0 shimming was performed using FASTESTMAP8 and MR spectra were acquired using the modified semi-LASER9 (TR/TE = 5000/28ms, 64 transients).
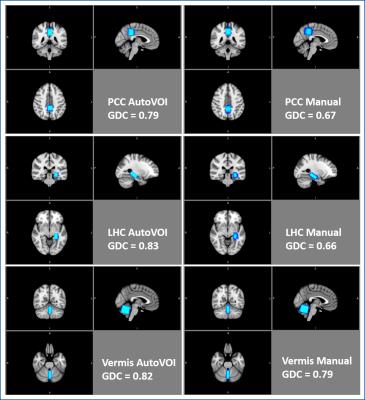
Spectra were processed with MRspa10 and fitted using LCModel11 with water scaling, as described previously7. In addition, all VOI placements were normalized to atlas space using Elastix12 and the spatial overlap was used to calculate the generalized Dice coefficients13 (GDC) to quantify spatial consistency of VOI placements.
Results
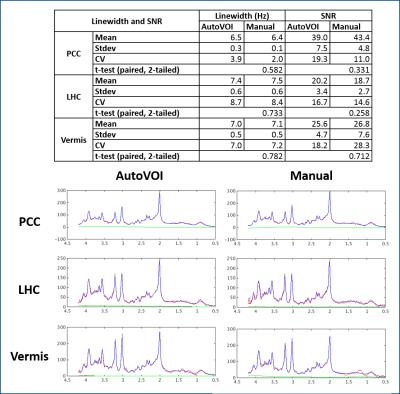
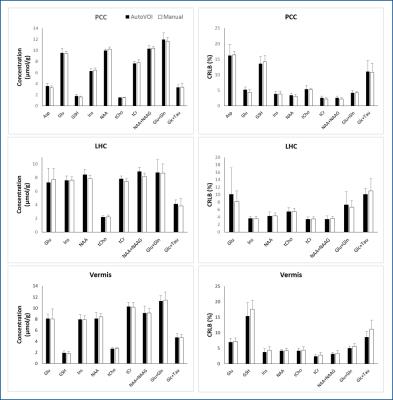
AutoVOI calculation for one voxel was performed under 30 seconds, and the whole VOI prescription process took about two minutes, including retrieval of DICOM files from the image database and network transmission of the data. This was comparable to manual placement where 3D re-slicing of image data was needed prior to manual placements. Linewidths and SNR in spectra obtained from automatically and manually placed voxels were comparable, resulting in similar spectral quality (Fig3). Consistently, no differences were observed in neurochemical concentrations obtained from manual and automatically placed VOI (p = 0.84 for PCC, 0.06 for LHC, 0.90 for vermis; paired, 2-tailed t-test) (Fig4). Higher GDC values were obtained with AutoVOI than manual VOI placement in all three VOIs demonstrating better across-subject spatial consistency with AutoVOI (Fig5).Discussion
Voxel prescription through AutoVOI was at least as accurate as manual VOI placement based on spectral quality and pattern obtained in VOI prescribed with the two methods. Furthermore, AutoVOI enabled higher inter-subject consistency in VOI prescription based on higher GDC values than manual placement in all three VOIs. This demonstrates that AutoVOI accommodates anatomical variability between subjects better than manual voxel prescription.Conclusion
This study demonstrated the feasibility and superiority of using AutoVOI over manual voxel prescription for inter-subject spatial consistency in MRS scans. We expect that automation of voxel prescription will lead to increased clinical adoption of MRS by reducing operator dependence and making it easier for MR technologists to acquire high quality MRS data.Acknowledgements
Supported by NIH R01 NS080816, R01 NS070815, P41 EB015894 and P30 NS076408.
Also supported by the Brain Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (2014M3C7033999), and by the grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry for Health and Welfare, Korea (HI14C1135).
References
1. Park YW, Deelchand DK, Joers JM, Soher BJ, Barker PB, Park HW, Öz G, Lenglet C. Fast automatic voxel positioning with non-rigid registrations for improved between-subject consistency in MRS. Proc. Intl. Soc. Mag. Reson. Med. 24 (2016)
2. O’Rourke J, Finding Minimal Enclosing Boxes. International Journal of Computer and Information Sciences, 1985;14(3):183-199.
3. Smith S M. Fast robust automated brain extraction. Human Brain Mapping. 2002;17(3):143-155.
4. Eklund A, Dufort P, Villani M, LaConte S. BROCCOLI: Software for fast fMRI analysis on many-core CPUs and GPUs. Frontiers in Neuroinformatics. 2014;8(24):1-19.
5. Bednarik P, Moheet A, Deelchand D K, Emir U E, Eberly L E, Bares M, Seaquest E R, Öz G. Feasibility and reproducibility of neurochemical profile quantification in the human hippocampus at 3T. NMR in Biomedicine. 2015;28:685-693.
6. Terpstra M, Cheong I, Lyu T, Deelchand D K, Emir U E, Bednarik P, Eberly L E, Öz G. Test-Retest Reproducibility of Neurochemical Profiles with Short-Echo, Single-Voxel MR Spectroscopy at 3T and 7T. Magn Reson Med. 2016;76(4):1083-1091.
7. Deelchand DK, Adanyeguh IM, Emir UE, Ngyuen TM, Valabregue R, Henry PG, Mochel F, Öz G. Two-site Reproducibility of Cerebellar and Brainstem Neurochemical Profiles With Short-Echo, Single-Voxel MRS at 3T. Magn Reson Med 2015;73:1718-1725.
8. Gruetter R, Tkác I. Field mapping without reference scan using asymmetric echo-planar techniques Magn Reson Med. 2000;43(2):319-324.
9. Öz G, Tkác I. Short-Echo, Single-Shot, Full-Intensity Proton Magnetic Resonance Spectroscopy for Neurochemical Profiling at 4 T: Validation in the Cerebellum and Brainstem Magn Reson Med. 2011;65(4):901-910.
10. Deelchand DK, MRspa: Magnetic Resonance signal processing and analysis, https://www.cmrr.umn.edu/downloads/mrspa/. Accessed November 2, 2016.
11. Provencher SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med. 1993;30(6):672-679.
12. Klein S, Staring M, Murphy K, Viergever M A, Pluim J P W. elastix: a toolbox for intensity based medical image registration. IEEE Trans on Medical Imaging. 2010;29(1):196-205.
13. Crum WR, Camara O, Hill DLG. Generalized Overlap Measures for Evaluation and Validation in Medical Image Analysis. IEEE Trans on Med Imag. 2006;25(11):1451-1461.
Figures