3020
4-Dimensional spin echo for prostate 1H MRSI at 7T using a multi-transmit system1Imaging Division, University Medical Center, Utrecht, Netherlands, 2Department of Physics and Medical Technology, VU University Medical Center, Amsterdam, Netherlands
Synopsis
A 4-dimensional spatially and spectrally selective spin echo sequence was developed for prostate 1H MRSI at 7T. This sequence has intrinsic water and lipid suppression properties due to the limited spectral bandwidth, so only prostate metabolites are measured. The low peak power of the pulses also leads to lower SAR deposition and RF duty cycles. Phantom experiments showed an average water suppression of 99.99% at short TE, with low SAR and RF duty cycle values compared to adiabatic pulses routinely used at higher field strengths. Combined, these properties enable fast prostate MRSI acquisitions without water and lipid artifacts.
Introduction
Prostate cancer (PCa) is the second most prevalent cancer in men worldwide1. Current diagnostic techniques are PSA screening and needle biopsy. However, PSA screening leads to overdetection2, while needle biopsy is invasive and limited in accurate determination of aggressiveness due to the multifocal and heterogeneous nature of PCa3. Therefore, a more accurate method to determine the aggressiveness of PCa is needed.
Proton MRSI is capable of detecting alterations in metabolite concentration (i.e. choline, polyamines, creatine and citrate) that play a role in prostate cancer. Higher field strengths, such as 7T, offer an increase in spectral resolution, enabling the individual detection of the prostate metabolite peaks, which overlap at lower field strengths.
Current prostate MRSI protocols are lengthy and require water and fat suppression schemes. Adiabatic pulses are commonly used at higher field strengths to compensate for their inherent RF field inhomogeneity, leading to elevated SAR and RF duty cycles. Here, we propose the use of two-dimensional RF pulses for volume pre-selection and refocusing with a limited spectral bandwidth, to allow simultaneous water and lipid suppression and detect only prostate metabolites. Additionally, this sequence uses lower peak power pulses, therefore lowering SAR deposition and RF duty cycles.
Methods
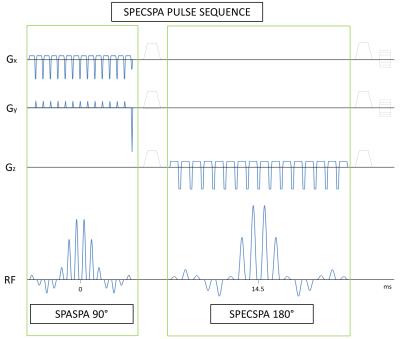
A 4D spatially and spectrally selective spin echo pulse sequence (SPECSPA) was developed. The excitation pulse consisted of 14 composites sinc pulses of 0.4096ms each, separated by 0.24ms in a sinc envelope of 8.8544ms total duration. Slice selective gradients were used to excite the first spatial direction. Blip gradients were positioned between each composite pulse to excite in the second spatial direction resulting in a 2D spatially selective pulse. The refocusing pulse consisted of 16 composite sinc pulses of 0.6528ms, separated by 0.35ms in a sinc envelope of 15.6948ms total duration. The third spatial direction was selected by slice selective gradients and the spectral selectivity was achieved by the sinc envelope of the spectral-spatial pulse. The refocusing pulse has a small spectral bandwidth (360Hz, 1.2ppm) and therefore has intrinsic water and fat suppression when centered at 2.8ppm leaving only the prostate metabolites. Echo time simulations were obtained for the strongly coupled spin system of citrate using the Python based VeSPA simulation 0.9.2 software (©2010, Duke University). An echo time (TE) of 29ms was chosen to have the citrate spin system in a semi-optimal absorptive mode to keep the TE short.
A double compartment phantom was created to test our SPECSPA sequence. The inner compartment of the phantom contained a solution mimicking prostatic fluid as described in Van Der Graaf et al.4. The outer compartment of the phantom contained a 50mM NaCl solution to match the body dielectric properties. The phantom size was comparable to the size of the human pelvic region at 25x40x16cm.
Measurements were done on a 7 Tesla Achieva system (Philips, Cleveland, OH, USA) using an 8-channel parallel transmit system. Eight fractionated dipole antennas5 were used as transceivers and every antenna had 2 additional receive loops. This array was positioned externally around the phantom. RF Phase shimming and B0 shimming were obtained prior to the MRSI acquisition (2D MRSI (SPECSPA, TE/TR=29/2000ms, 5x5x1 matrix, 10x10x30mm voxel, 50x50x30mm FOV, acquisition time=38s, B1max=11.4μT, 2.5x2.5x30mm recon voxel)).
Results and discussion
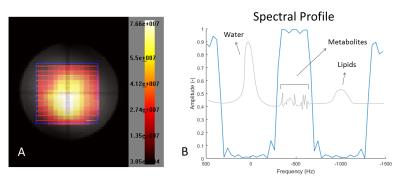
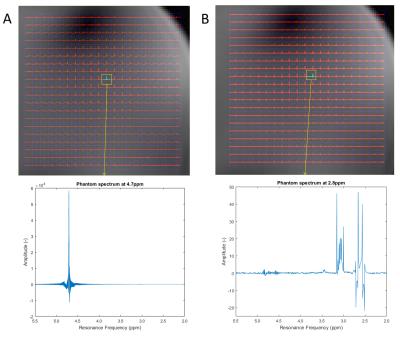
Figure 1 shows the resulting 4-dimensional spin echo sequence. Figure 2a shows the resulting spatial-spatial profile from the excitation pulse. Figure 2b shows the spectral profile of the refocusing spectral-spatial pulse with a refocusing BW of 360Hz, enough to include only the prostate metabolites of interest and suppress water and lipids simultaneously. Notice the excellent (99.99% on average) water suppression obtained, as shown in Figure 3, and the presence of prostate metabolites in the phantom. Since the SPECSPA sequence uses conventional RF pulses with a maximum RF power of 11.4μT, this resulted in a maximum local SAR of 0.125W/kg and an RF duty cycle of 1.2%. After RF shimming, B1 variations within the volume of interest (7-12μT) were still observed. However, this had no major influence in the excitation nor the refocusing profiles.Conclusion
A 4-dimensional spin echo sequence was successfully developed for obtaining MR spectroscopy of the prostate in combination with an 8-channel multi-transmit system. Excellent water suppression and short-TE spectra can be obtained with this sequence at low SAR and duty cycle values. This enables fast prostate MRSI acquisition.Acknowledgements
No acknowledgement found.References
1. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global Cancer Statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108.
2. Schröder FH, Hugosson J, Roobol MJ, Tammela TLJ, Ciatto S, Nelen V, et al. Screening and prostate-cancer mortality in a randomized European study. N Engl J Med. 2009;360(13):1320–8.
3. Cookson MS, Fleshner NE, Soloway SM, Fair WR. Correlation between Gleason score of needle biopsy and radical prostatectomy specimen: Accuracy and clinical implications. J Urol. 1997;157(2):559–62.
4. Van Der Graaf M, Schipper RG, Oosterhof GON, Schalken JA, Verhofstad AAJ, Heerschap A. Proton MR spectroscopy of prostatic tissue focused on the detection of spermine, a possible biomarker of malignant behavior in prostate cancer. Magn Reson Mater Physics, Biol Med. 2000;10(3):153–9.
5. Raaijmakers AJE, Italiaander M, Voogt IJ, Luijten PR, Hoogduin JM, Klomp DWJ, et al. The fractionated dipole antenna: A new antenna for body imaging at 7 Tesla. Magn Reson Med. 2016;75(3):1366–74.
Figures