3017
Very Short Echo Time MRS for Single Voxel Spectroscopy in Small Voxels1Physikalisch-Technische Bundesanstalt (PTB), Berlin, Germany, 2Max Planck Institute for Biological Cybernetics, Tuebingen, Germany, 3Center for Stroke Research Berlin (CSB), Charité - Universitätsmedizin Berlin, Berlin, Germany, 4Institute of Physics, Ernst-Moritz-Arndt University Greifswald, Greifswald, Germany
Synopsis
This work presents the combination of metabolite cycling with a non-water-suppressed SPECIAL localization scheme in order to enable improved averaging coherence for very short echo time MRS in small voxels or voxels that suffer otherwise from low SNR or frequency instabilities.
Introduction
Metabolite cycling1 in combination with PRESS, MEGA-PRESS, STEAM and semiLASER localization schemes has been shown to enable the acquisition of high quality single voxel spectra from small voxels, at high field, and from challenging regions2,3,4,5,6. This non-water-suppressed spectroscopy technique allows for retrospective phase and frequency correction using the water peak as a strong reference signal, where metabolite signals are too small for this task, and hence, enable more coherent averaging. This is particularly important for small voxels and multi-step localization. However, PRESS is limited to rather long echo times. Hence, by the time the echo is sampled with a PRESS sequence, the signal amplitudes of metabolites with short relaxation times have decayed and are no longer detectable. STEAM sequences, on the other hand, allow very short echo times, but suffer from a two-fold signal loss compared to PRESS. In 2006, the two-step SPECIAL localization scheme was introduced, which allows MRS with very short echo times at full signal amplitude7.
This work presents the combination of metabolite cycling with a non-water-suppressed SPECIAL localization scheme in order to enable improved averaging coherence for very short echo time MRS in small or low SNR voxels.
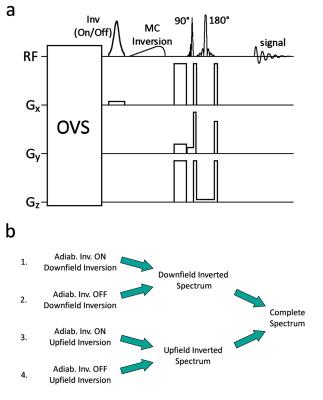
Pulse Sequence
In
the SPECIAL localization scheme7,8 the magnetization of a slice is
inverted in alternating acquisitions, and a slice selective spin echo sequence,
orthogonal to the first slice, is applied. An add/subtract scheme according to the application of the inversion pulse, leads then to full localization. The sequence proposed here contains an additional semi-adiabatic frequency selective
pulse between the first inversion pulse and the excitation pulse
(Fig.1a). This pulse inverts the magnetization of the metabolites either upfield
or downfield of water in alternating full SPECIAL acquisitions without
affecting the water. Subtraction of a fully localized downfield inverted
spectrum from a fully localized upfield inverted spectrum generates a metabolite spectrum(Fig.1b).Methods
MRS measurements were performed in 2 healthy volunteers at a 3T Verio system (Siemens Healthcare,Erlangen, Germany) equipped with a 32 channel head coil. For voxel positioning a T1 weighted MPRAGE image was acquired. A localized RF calibration and automated 2nd order FAST(EST)MAP9,10 B0 shimming were performed prior to spectroscopy measurements.
First, to validate the sequence, spectra from a medium-sized voxel (20×20×20mm3) in the posterior cingulate cortex (PCC) were acquired (TE=8.5ms, TR=3s, 64 averages), once with a regular SPECIAL sequence and once with the proposed MC-SPECIAL sequence with otherwise identical acquisition parameters. In addition, one spectrum from three different smaller voxels (10×10×10mm3) placed in the grey matter of 1) PCC; 2) middle temporal lobe (MTL); and 3) anterior cingulate cortex (ACC) were acquired with both sequences (TE=8.5ms, TR=3s, 128 averages).
An in-house developed software was used for coil combination, frequency correction (where possible), adding and subtracting the acquisitions appropriately, and finally averaging. LCModel11 was used to fit the data.
Results and Discussion
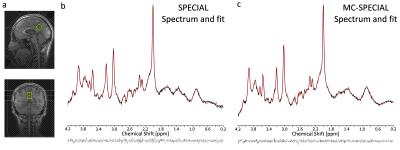
Fig.2 shows the spectrum and fit of the spectrum acquired in the medium-sized PCC voxel with the regular SPECIAL sequence and with the proposed MC-SPECIAL sequence. Both spectra are very similar in appearance and quality.
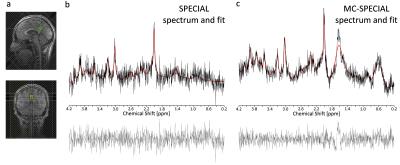
An individual SPECIAL acquisition does not provide sufficient SNR to perform frequency correction, when processing the spectra acquired in the small PCC voxel. In contrast, in the non-water-suppressed MC-SPECIAL measurement the large water peak of each acquisition can be used as reference for frequency correction, resulting in enhanced SNR. Fig.3 displays the fitted spectra comparing non-frequency-corrected SPECIAL with MC-SPECIAL. An SNR enhancement in the MC-SPECIAL spectrum compared to the SPECIAL spectrum is clearly visible. A lipid artifact12 becomes apparent in the spectra acquired from the small voxels. The origin and effective suppression of this, will be subject of future studies.
The SNR in the data acquired with SPECIAL in the MTL and ACC voxels was higher than in the PCC voxel, therefore the difference in spectral quality between SPECIAL and MC-SPECIAL measurements is not as pronounced as in the data shown here.
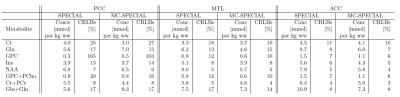
Tab.1 compares the concentrations of 8 metabolites for the two different acquisition sequences in the three different voxel positions. The calculated concentrations obtained with MC SPECIAL differ from those acquired with SPECIAL from the same voxel. This difference might be attributed to magnetization transfer effects, which is reduced in water-suppressed measurements. However, this question requires further investigation.
Conclusion
This work demonstrates for the first time the feasibility of the combination of non-water-suppressed metabolite cycling with a SPECIAL localization scheme. This enables very short echo time spectra from small voxels that may have very low SNR, to be efficiently frequency shift corrected. Thus, short lived metabolite signals can be detected with improved spatial specificity.Acknowledgements
The authors greatly appreciated the very short notice help with the solution of IDEA compiling problems of Dr. Sebastian Schmitter and Dr. Christoph Kolbitsch.References
[1] Dreher W. et al., Magn Reson Med 2005;54(1):190-195
[2] MacMillan E.L. et al., Magn Reson Med 2011;65:1239–1246
[3] Hock A. et al., Magn Reson Med 2012; 69 :1253-1260
[4] Zoelch N. et al., Proc Intl Soc Magn Reson Med 2014;22:66
[5] Fillmer A. et al., Proc Intl Soc Magn Reson Med 2016;24 :1100
[6] Giapitzakis I.A. et al., Proc Intl Soc Magn Reson Med 2014 ;22 :2895
[7] Mlynárik V. et al., Magn Reson Med 2006 ;56 :965-970
[8] Mekle R. et al., Magn Reson Med 2009 ;61 :1279-1285
[9] Gruetter R. et al., J Magn Reson 1992;96:323–334
[10] Gruetter R. et al., Magn Reson Med 2000;43:319–323
[11] Provencher S. W. Magn Reson Med 1993;30(6):672-679
[12] Fuchs A. et al. Magn Reson Med 2013;69:603-612
Figures