2989
NMR phytometabolomics for evaluation of non-polar chemosensory signatures1Department of NMR, All India Institute of Medical Sciences, New Delhi, India
Synopsis
With increasing interest in the role of taste related fatty acids in food, pharmacology, health and diseases, objective study of this chemosensory parameter has assumed importance. The potential of NMR metabolomics to fingerprint chemosensory properties has been explored in the context of non-polar phytocompounds in this study. Non-polar fractions obtained by dual phase (chloroform-methanol/water) extraction of select dietary plants (n=24) were studied using proton NMR spectroscopy. Partial Least Squares Discriminant Analysis of the spectral data showed distinct chemosensory based clustering. NMR based chemosensory studies of non-polar phytocompounds could open new applications in sensorial sciences related to lipids and fatty acids.
PURPOSE
The chemosensory property of taste is no more restricted to only taste buds since taste receptors are now known to be present in tissues outside the oral cavity, for example in digestive and respiratory systems1. Their roles in regulating various physiological processes are currently a matter of indepth study1. At the same time, recent studies have provided compelling evidence of fat as a sixth taste2. The chemoreception of lipids and fatty acids, their physiological consequences, their impact on health and implications for diseases such as obesity are also gaining much attention2. All these have renewed interest and widened the scope of chemosensory properties of dietary ingredients and their role in diseases. Applications of NMR in sensory sciences have long been an area of research interest. The present work explores the role of NMR metabolomics of non-polar phytocompounds of dietary and nutraceutical plants from different chemosensory groups.
METHODS
Extraction of non-polar phytocompounds: Twenty four plants from four chemosensory categories (sweet, astringent, bitter and pungent) were studied. Some of the plants examined were Phoenix sylvestris, Vitis vinifera, Cocos nucifera, Ficus racemosa, Momordica charantia, Piper nigrum and Piper longum. The plant parts were subjected to dual phase (chloroform-methanol/water) extraction to separate the polar from the non-polar compounds. The extracts were dried using rotary vacuum concentrator to remove the solvent.
NMR studies: The non-polar compounds were dissolved in CDCl3 containing 0.1% TMS, which served as the reference. 1D Proton NMR spectra were acquired at 700 MHz (Agilent, USA) using the following parameters: relaxation delay - 14 sec, no. of scans - 64, spectral width - 12 ppm, data points - 32 K. Peak assignments were carried out using NMRShift database library.
Data analysis: The NMR data were binned and bucketed at intervals of 0.04 ppm for Partial Least Squares Discriminant Analysis (PLS-DA) (Metaboanalyst 3.0). Spectral data post-processing, binning and bucketing were carried out using MestReC.
RESULTS
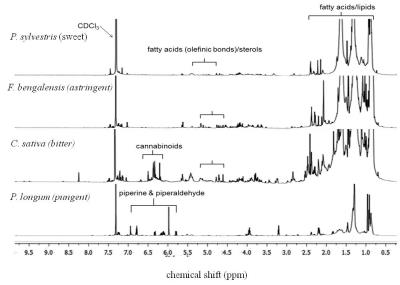
Figure 1 shows
representative spectra from non-polar fraction of plants from different taste
categories. Non-polar phytometabolites like fatty acids, lipids and sterols are
seen in the spectra. It is interesting to see taste associated metabolites like
piperidine alkaloids in the spectrum of Piper
longum. PLS-DA of the
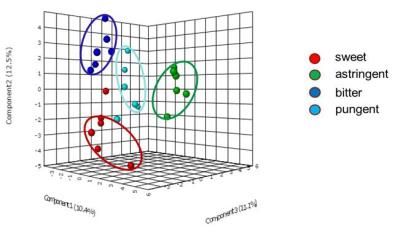
spectral data (Fig. 2) showed distinct differences between all the taste groups,
indicating similarity in the compounds extracted from plants of same group. The results also indicate that non-polar
compounds may have significant role in the chemosensory properties of these
studied plants.CONCLUSION
Accumulating evidences indicate the diverse roles taste plays in nutrition, pharmacology, health and also diseases. The intriguing observation of fat as the sixth taste and its role in diseases such as obesity has aroused much interest in its objective analysis. In particular, the potential relationship between fatty acids and disease makes this a parameter of interest. Taste being a chemical sense, lends itself to studies by NMR, which is ideally suited to look at complex sample matrix without isolation of compounds. In this first time exploratory NMR study of non-polar dietary phytocompounds, distinctive NMR spectral attributes that allow chemosensory-based differentiation of these compounds were observed. The results lead to speculations on directions for further research such as exploring candidates for gustatory lipid sensation. Such studies can serve as vital inputs to information on chemoreception of lipids and their physiological impact on health and diseases.Acknowledgements
The work was supported by National Medicinal Plants Board, Ministry of AYUSH, Govt. of India.References
1. L Feng. Taste perception: from the tongue to the testis. Mol Human Reprodn, 19, 349-360, 2013
2. Besnard P, Passilly-Degrace P, Khan NA. Taste of Fat: A Sixth Taste Modality? Physiol Rev, 96, 151-176, 2016.