2988
Metabolic Assessment of a Migraine Model using Relaxation-Enhanced 1H Spectroscopy at Ultra-High Field1Center for Interdisciplinary Magnetic Resonance, National High Magnetic Field Laboratory, Tallahassee, FL, United States, 2Chemical & Biomedical Engineering, Florida State University, Tallahassee, FL, United States, 3Department of Chemical Physics, Weizmann Institute of Science, Rehovot, Israel, 4Molecular Neurology Program, Huntington Medical Research Institutes, Pasadena, CA, United States
Synopsis
This study evaluates biochemical and metabolic imbalances that may result in a collection of dysfunctional pathways that are distinct in migraineurs. The high sensitivity and spectral dispersion available to 1H MRS at 21.1 T expanded metabolic profiling in an animal model of migraine to include total creatine (tCr), choline (Cho), N-acetyl-aspartate (NAA), myoinositol (mI), lactate (Lac), taurine (Tau), aspartate (Asp), Glx, a mixture of glutamate, glutamine and GABA, and Gly, the latter identified as a mixture of glycine, glutamine, and glutamate. For the migraine analogue, Lac, Gly and Tau increased while tCr decreased temporally and in comparison to saline controls.
Purpose
High-field MR spectroscopy provides the opportunity to interrogate metabolites dynamically and in vivo during the progression of neurological disorders, such as migraine, that have rapid onset but potentially longer impacts. It thus follows that identification of specific metabolic changes, potentially influenced by ionic imbalances or the influence of excitatory and inhibitory neurotransmitters, may improve understanding of the onset and progression of migraine1 and open up new avenues for therapy development. This study evaluates biochemical and metabolic imbalances that may result in a collection of dysfunctional pathways that are distinct in migraineurs. The high sensitivity and spectral dispersion available to 1H MRS at 21.1 T expands metabolic profiling of the migraine model from conventional small molecules (total creatine (tCr), choline (Cho), N-acetyl-aspartate (NAA), Glx—a mixture of glutamate, glutamine and GABA) to include lactate (Lac), taurine (Tau), myoinositol (mI), aspartate (Asp), and Gly, the latter identified as a mixture of glycine, glutamine and glutamate.Materials and Methods
Animal Model: To induce a migraine analogue in rodents, 17 anesthetized Sprague-Dawley male rats were administered an in situ intraperitoneal injection of either nitroglycerine (NTG, N=11) to induce central sensitization (CS) related to migraine or saline (N=6) as a control.
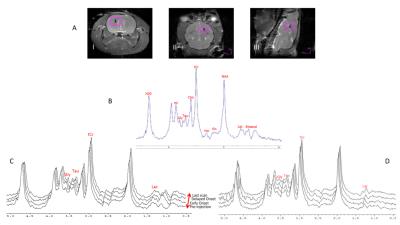
MRS protocol: All scans were performed using the 21.1-T, 900-MHz ultra-wide bore at the National High Magnetic Field Laboratory, Tallahassee, FL. A home-guilt linear 1H/23Na birdcage coil was used for all acquisitions. A highly selective Relaxation Enhanced MRS (RE-MRS)2 sequence was utilized to target upfield metabolites from a (4-mm)3 voxel, without water suppression. Selective excitation was accomplished with a 5-ms 10-lobe, sinc-shaped pulse, and a 5-ms 180° refocusing pulse derived from the Shinnar-LeRoux algorithm (SLR), exciting a band between 0-4 ppm without touching the water resonance. 3D spectroscopic localization was achieved using six 5-ms adiabatic pulses with localization by an adiabatic selective refocusing (3D LASER) methodology. A total of 14 scans (10 min/scan) were acquired from pre-injection to 2.5-h post injection.
Data Analysis: Data was acquired as a partial echo, and magnitude spectra were generated after apodization (10-Hz exponential line broadening) and Fourier transform. No modeling or deconvolution of spectral components was performed. This raw data time course was normalized to the NAA peak (found experimentally to be static in NTG and saline over all data points) and was further referenced to the pre-injection value. Individual time points from all animals in the NTG and saline groups based on these time series were averaged to determine trends and significance. A mixed model ANOVA with repeated measures was used to conduct the with-in subject and between subject analysis.
Results
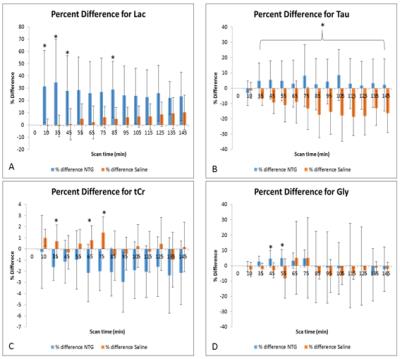
The RE-MRS sequence provides high fidelity data with flat baselines while avoiding the water resonance through the selective excitation of the targeted metabolites upfield of the bulk water resonance (Figure 1). Significant increases were evident for Lac as early as 10 min post NTG injection, peaking at 35 min at over 50% compared to baseline and control (Normalized Lac/NAA with NTG = 1.54±0.65 vs. with saline = 0.99±0.08). Tau decreased in controls progressively over 2-h post-injection but remained elevated with NTG, peaking at 105 min post NTG injection (Normalized Tau/NAA with NTG = 1.10±0.18 vs. with saline = 0.85±0.14). tCr under NTG showed significant decreases both with time and saline; Gly demonstrated increases with time for NTG. Figure 2 is reflective of these impacts.Discussion
These metabolic changes (rapid increase in lactate with a subsequent relative increase in taurine compared to controls) indicate that an altered metabolic profile results from NTG. The rapid elevation of lactate may reflect increased neural activity and associated ionic imbalances, alteration in glucose metabolism and/or vasodilation at the early stage of CS in the migraine analogue. The elevated levels of taurine may be evidence of reactive neuroprotective and osmoregulatory mechanisms. These metabolic substrates reflect the physiological cascade involved in CS. Elevated lactate and taurine may be biomarkers in future studies to investigate chronic migraine progression and intervene clinically.
Conclusions
The present study is novel as it is the first time that metabolic substrates have been evaluated in the living brains of an acute rodent model on a time course basis. Acute metabolic alterations and targets were identified as both substrate and time dependent, and could provide insight into dysregulation during CS.Acknowledgements
This work was supported by the NIH (R01-NS072497 to MGH) and User Collaborations Grant Program (to SCG) from the National High Magnetic Field Laboratory, which is funded by the NSF (DMR-1157490) and the State of Florida.References
1. Harrington M, Fonteh A, Cowan R, Perrine K, Pogoda J, Biringer R, Huhmer A. Cerebrospinal fluid sodium increases in migraine. Headache 2006;46(7):1128-35.
2. Shemesh N, Rosenberg JT, Dumez J, Muniz JA, Grant SC, Frydman L. Metabolic properties in stroked rats revealed by relaxation-enhanced magnetic resonance spectroscopy at ultrahigh fields. Nat Commun 2014;5:4958.
Figures