2985
Experimental setup for direct observation of hippocampal glycolysis in rat brain as a marker of cellular health1RG Translational Imaging, Department NeuroImaging, Central Institute of Mental Health, Medical Faculty Mannheim, University of Heidelberg, Mannheim, Germany, 2mfd diagnostics GmbH, Wendelsheim, Germany
Synopsis
Glycolysis is fundamental for cerebral energy metabolism. The current glycolysis pathway gives direct information about the local cellular health. To investigate hippocampal glycolysis in living rats, we established an experimental setup combining MRS and laser spectroscopy. It provides the opportunity to observe the behavior to the involved substances (glucose, lactate, NADH) for different glycolytic pathways. Under hyperglycemic conditions, blood oxygen limits glucose consumption leading to drops in NADH and lactate. In contrast, NADH and lactate concentrations increase under anaerobic conditions. This corresponds well with known literature and demonstrates the power of the presented setup to characterize hippocampal glycolysis in vivo.
PURPOSE
Cerebral energy metabolism is based on glycolysis. Its current pathway depends on demand of energy, resources of glucose and oxygen, and cellular environment. Therefore, observing glycolysis gives direct information about local cellular health. The purpose of this study was to establish an experimental setup investigating hippocampal glycolysis in living rats. The presented setup combines methods of magnetic resonance spectroscopy (MRS) and laser spectroscopy (LS). Hence, we were able to directly manipulate the extracellular fluid composition as well as monitor the local cerebral glycolysis.METHODS
Two Sprague-Dawley rats (~185g) were anesthetized initially by a gas mixture of O2: 20% and air: 80% with ~4% isoflurane. For each rat, an optical fiber was implanted together with an infusion pipe in the hippocampus. Afterwards, continuous laser spectroscopic (LS) measurements of NADH fluorescence (NADHJA®, MFD Diagnostics GmbH, Wendelsheim, Germany) combined with magnetic resonance spectroscopy (MRS) were performed on a 9.4T Bruker Biospec system. For MR signal generation the conventional setup using a 4 channel receiver array and a volume transmit coil were used. For MRS, PRESS pulse sequence was applied several times to detect the signal response of glucose and lactate while manipulating the glycolysis (TR/TE = (4000/10)ms , dwell time = 124.8us, bandwidth = 4006Hz, 256 averages, 17min4s measurement time). During the MRS measurements, isoflurane rate was kept around 2% ensuring a constant respiration rate of 65-75bpm. Figure 1 shows the positions of the implanted LS fiber and the MRS voxel in the rat brain.
The protocol for induced manipulation of hippocampal glycolysis is shown in figure 2. After recording a reference MRS spectrum, local extracellular hyperglycemia was induced per glucose infusion (0.4g glucose in 3ml NaCl; 1ul/min) over 30 minutes, and after further 30 minutes an overdose of isoflurane (5%) was applied until circulatory collapse. The measured individual MRS spectra were combined, and averaged spectra for every ~8,5min were computed applying the sliding window technique (increment of 128 spectra, averaging of 256 spectra). The generated spectra were quantitatively analyzed with LCModel (range 0.5-4.0ppm).
RESULTS
Figure 3 show MR spectra during several conditions. Results of MRS and corresponding LS measurements over time for both animals are presented in Figure 4. During infusion, glucose accumulated in the hippocampus with no influence on respiration rate, whereas NADH and lactate dropped. After administrating an overdose of isoflurane and subsequent circulatory collapse, glucose concentration returned to the reference concentration for one animal, for the second one it dropped below limit of detection. In contrast, lactate and NADH rose steeply to an individual level.DISCUSSION
In this study, we introduced an experimental setup to characterize different glycolytic pathways. Three pathways were investigated: normal oxidative glycolysis, glycolysis during hyperglycemia, and glycolysis during lack of oxygen after circulatory collapse. The normal oxidative glycolytic pathway served as a reference for the other two induced conditions. During hyperglycemic condition, we found that in cells generation of energy using glucose is only limited by the available blood oxygen. This leaded to a drop in the intracellular NADH concentration, which is involved in the glycolytic pathway. Correspondingly, production of lactate was reduced due to the hyperglycemic condition resulting in a diminished concentration. After the circulatory breakdown the oxygen supply was stopped, leading to accumulation of lactate as well as unused NADH during this anaerobic glycolytic condition. Our findings are in accordance with known literature. By this, the presented experimental setup is supported as a suitable tool to investigate the hippocampal response during manipulation of local glycolysis.CONCLUSION
In this study, we established an experimental setup for the characterization of hippocampal glycolytic pathways under different induced conditions in living rats.Acknowledgements
This study was funded by "The Central Innovation Programme (ZIM)" of the Federal Ministry for Economic Affairs and Energy, Germany.References
No reference found.Figures
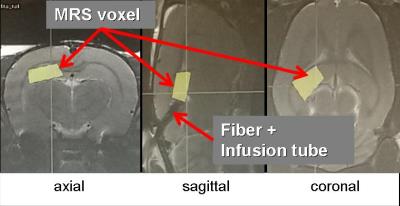
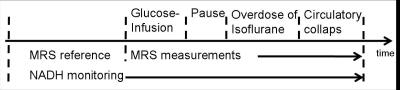
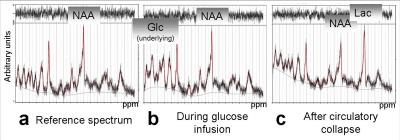
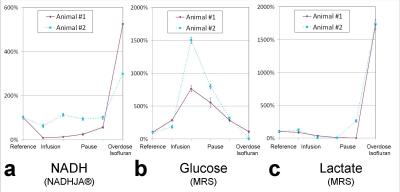
Figure 4: Depiction of a) NADH, b) glucose, c) lactate over time for both investigated animals. After reference measurement, NADH and lactate showed correspondingly a dip during glucose infusion, whereas glucose increased. After administration of an overdose of isoflurane, NADH and lactate rose steeply. In contrast, glucose returned to reference concentration for one animal and dropped under level of detection for the other.