2982
Brain Metabolite Level Estimation at Anterior Cingulate Cortex in Fibromyalgia Using Advanced MRS1RADIOLOGY, TAIPEI VETERANS GENERAL HOSPITAL, Taipei, Taiwan, 2School of Medicine, National Yang-Ming University, Taipei, Taiwan, 3School of Biomedical Engineering and Technology, Faculty of Imaging and Radiological Sciences, National Yang-Ming University, Taipei, Taiwan, 4NEUROLOGY, TAIPEI VETERANS GENERAL HOSPITAL, Taipei, Taiwan, 5GE Healthcare, Taipei, Taiwan, 6MR Research China, GE Healthcare, Beijing, People's Republic of China
Synopsis
We apply MEGA-PRESS MRS to measure brain metabolite level of Fibromyalgia patients and healthy controls at anterior cingulate cortex. The results show higher levels of GABA in Fibromyalgia than healthy controls but no significant difference was found in glutamine and glutamate levels.
Purpose
Fibromyalgia (FM) is a complex chronic pain disorder and is known to cause significant distress and disability in patients 1,2. An understanding of how the brain metabolite changes in chronic pain such as FM is important for setting therapeutic plan. Recent scientific findings have great interest in examining the role of γ-aminobutyric acid (GABA), the major inhibitory central nervous system neurotransmitter, in chronic pain conditions 3. The purpose of this study was to identify the nature of the cerebral dysfunction by assessing the brain metabolite patterns of GABA, glutamate (Gln) and glutamine (Glu) in patients with FM through advanced magnetic resonance spectroscopy (MRS) technique.Methods
Six FM diagnosed patients and five age-matched healthy controls (HC) were recruited in this study. All MRI acquisitions were performed on a 3T clinical scanner (Discovery MR750, GE Healthcare, Milwaukee, USA) using a body coil as RF excitation and an 8-chanel head coil as signal receiver. A 2.4×2.4 ×2.4 cm3 voxel of interest (VOI) was placed at anterior cingulate cortex (ACC) (Fig. 1) The voxel placement was chosen based on our previous experience on post spinal injury pain MRS study. The 1H spectrum optimized for detecting GABA, Gln, and Glu was acquired by a spectral editing MEGA-PRESS 4 sequence with the following parameters: TE/TR = 68/2000 ms; number of points= 2048; spectral width =2000 Hz; number of average = 160 (scan time = 11.92 min). The metabolite levels were analyzed using LCModel 5. We next perform t-test to compare metabolite levels between FM patients and healthy controls. P values less than or equal to 0.05 were considered significant.Results
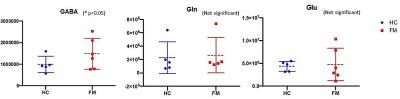
A representative proton MRS editing spectrum from the ACC and the fitting result of metabolite peaks using LCModel is shown as Figure 2. The peak for the combined measure of Gln and Glx is resolved at 3.8 ppm, and the peak for GABA is resolved at 3.0 ppm, with an inverted N-acetylaspartate (NAA) peak at 2.0 ppm. The minimal residual signal was found which expresses the good fit of metabolite peaks. In the quantitative data (Figure 3), FM patients demonstrated higher level of GABA (mean ± SD, 9.8x105 ± 4x105 AIU) compared to healthy controls (1.4x106 ± 5.5x105 AIU; P = 0.049). There was no significant differences in the mean level of Gln and Glx between FM patients and HCs, though the elevated trend was found.Discussion: and Conclusion
Higher level of GABA was found in FM than HC at ACC. The finding appeared to be similar with previous study1 which showed the same trend toward elevated concentrations, though no significant was observed. In conclusion, we find that GABA alteration in the ACC is a potential pathologic mediator in FM. Prior findings indicating the presence of allodynia and hyperalgesia in these patients may therefore be explained by the alteration in inhibitory neurotransmission within the ACC.Acknowledgements
No acknowledgement found.References
1. Foerster BR, Petrou M, Edden RA, et al. Reduced insular gamma-aminobutyric acid in fibromyalgia. Arthritis Rheum 2012; 64: 579–583.
2. Reckziegel D, Raschke F, Cottam WJ, Auer DP. Cingulate GABA levels inversely correlate with the intensity of ongoing chronic knee osteoarthritis pain. Molecular Pain. 2016;12: 1744806916650690.
3. Jasmin L, Rabkin SD, Granato A, Boudah A, Ohara PT. Analgesia and hyperalgesia from GABAmediated modulation of the cerebral cortex. Nature. 2003; 424:316–20.
4. Mescher, M., Garwood, M., Gruetter R., et al., Simultaneous in vivo spectral editing and water suppression, NMR Biomed (1998);11:266–272.
5. Stephen Provencher, Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med 30, 672 (1993).
Figures