2981
7 Tesla 1H MR Spectroscopy of the Motor Cortex following Transcranial Direct Current Stimulation1Centre for Functional and Metabolic Mapping, Robarts Research Institute, London, ON, Canada, 2Medical Biophysics, Western University, London, ON, Canada, 3Physics and Astronomy, Western University, London, ON, Canada, 4Clinical Neurological Sciences, Division of Neurosurgery, London Health Sciences Centre, London, ON, Canada
Synopsis
Transcranial direct current stimulation (tDCS) is a form of non-invasive brain stimulation that has been used to treat numerous cognitive and motor disorders. However, its mechanism of action is poorly understood, resulting in controversy over its effectiveness. The current study used ultra-high magnetic field (7 Tesla) magnetic resonance spectroscopy to determine if metabolite ratios were altered after the application of tDCS. In this preliminary study of 8 subjects, we found no differences in metabolite ratios in the motor cortex immediately following stimulation.
Purpose
Transcranial direct current stimulation (tDCS) is a controversial form of non-invasive brain stimulation. Despite claims of little to no effect on the brain1, the application of tDCS has been shown to increase cortical activity.2 In particular, tDCS has been associated with altered behaviour.2-6 Electrodes are placed on the scalp and pass a low, continuous current to selected brain regions, causing depolarization of the resting membrane potential. However, the exact mechanism of action, both during and after stimulation, and its associated effects on motor and cognitive faculties are unknown. Ultra-high magnetic field (7 Tesla) magnetic resonance spectroscopy (MRS) is a non-invasive tool that may be sensitive to tDCS induced changes in metabolite levels and could help elucidate the underlying mechanisms involved in applying tDCS to the brain. The goal of the current study was to determine whether metabolite levels were altered in the motor cortex immediately following bihemispheric tDCS applied to the motor areas of the brain.Methods
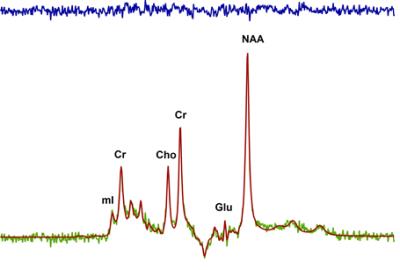
Eight healthy adults aged 21-40 participated in two sessions on a 7 Tesla Siemens MAGNETOM, head-only MRI, using an 8 channel transmit and 32 channel receive coil array. All participants had 1H MRS in this single blind, sham controlled, cross-over design. Participants were randomized to receive tDCS stimulation or sham stimulation on their initial visit, and the contrary on their second visit, at least 7 days apart. tDCS consisted of 2mA current applied for 20 minutes using an MR-compatible DC-STIMULATOR (neuroConn GmbH , Germany) to bihemispheric motor areas (cathode on left primary motor cortex, anode on right supplementary motor area) within the scanner. Temperature was monitored on all subjects to ensure safety. Four 0.60 mm diameter fibre optic temperature sensors (Neoptix, Quebec, Canada) were located under both electrode pads and the nearest cable chokes. Temperature was monitored in real time with a calibrated Reflex signal conditioner (Neoptix, Quebec, Canada) and a custom data collection program written in LabVEW 2010. Water suppressed (64 averages) and unsuppressed (8 averages) 1H MRS was acquired from the left primary motor cortex using a single voxel, semi Localization by Adiabatic Selective Refocusing (semi-LASER) pulse sequence: TE/TR = 60/7500 ms, voxel size=1.6x2x1.8 cm3. A localized B0 and B1 shim were applied to the voxel prior to data acquisition. In this preliminary analysis, the ratio of metabolites were normalized to creatine. Spectra were lineshape corrected using a combined QUALITY deconvolution and eddy current correction (QUECC).7 Spectra were fitted using prior knowledge of metabolite lineshapes in the fitMAN software (Figure 1).8 Metabolites measured with a coefficient of variation <30% were included for comparison. MRS acquisition began immediately following stimulation, capturing any alterations in metabolite ratios as a result of tDCS. Paired t-tests were performed between sham and stimulation with a significance set to p<0.05.Results
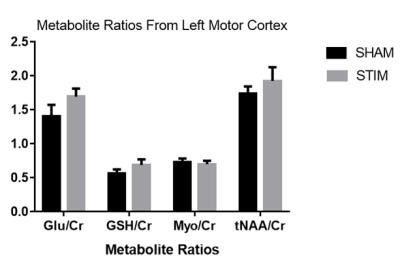
tDCS was successfully applied in the 7T MRI scanner in all subjects. Average temperature change in all four probes was 0.40±0.61 ºC during the semi-LASER sequence. Figure 1 shows a typical semi-LASER 1H spectrum acquired in one subject with the fitted result and residual. In this small sample, there were no significant differences between any of the metabolite ratios measured immediately following the sham and stimulation conditions (Figure 2)Discussion
Metabolite levels in the brain can be altered in a variety of neurological disorders. Recently it has been proposed that the application of tDCS may alter metabolites both during and after stimulation.9-11 The after effects of tDCS are thought to increase synaptic plasticity through modulation of the NMDA glutamate receptor.2 Glutamate has been shown to increase in concentration during and following tDCS.9,10 With the various protocols employed by different studies (intensity, duration, montage), it is difficult to draw conclusions about the effectiveness of tDCS, making its use controversial. To our knowledge, the current study is the first to combine ultra-high field (7T) MRI and concurrent tDCS stimulation. Although this preliminary study is underpowered, the bihemispheric montage of 2 mA of direct current stimulation for 20 minutes did not produce a measurable alteration in metabolite ratios to creatine immediately following the termination of stimulation. While previous studies have shown a change in glutamate concentration in the brain during stimulation, others have shown no significant after effects consistent with our results, which may be related to the intensity, duration and electrode montage employed.10Conclusion
The current study demonstrated no alteration in metabolite ratios following 20 minutes of tDCS. Although tDCS has been shown to alleviate the symptoms of many cognitive and motor disorders, it is unclear what physiological change is occurring. Repeated sessions of tDCS may be necessary to induce metabolic changes.Acknowledgements
Funding provided by Schulich School of Medicine, Western UniversityReferences
1. Horvath JC, Forte JD, Carter O. Evidence that transcranial direct current stimulation (tDCS) generates little-to-no reliable neurophysiologic effect beyond MEP amplitude modulation in healthy human subjects: A systematic review. Neuropsychologia 2015; 66: 213-36.
2. Nitsche MA, Paulus W. Transcranial direct current stimulation--update 2011. Restorative Neurology and Neuroscience 2011; 29(6): 463-92.
3. Antal A, Polania R, Schmidt-Samoa C, Dechent P, Paulus W. Transcranial direct current stimulation over the primary motor cortex during fMRI. NeuroImage 2011; 55(2): 590-6.
4. Boros K, Poreisz C, Münchau A, Paulus W, Nitsche MA. Premotor transcranial direct current stimulation (tDCS) affects primary motor excitability in humans. European Journal of Neuroscience 2008; 27(5): 1292-300.
5. Waters-Metenier S, Husain M, Wiestler T, Diedrichsen J. Bihemispheric transcranial direct current stimulation enhances effector-independent representations of motor synergy and sequence learning. Journal of Neuroscience 2014; 34(3): 1037-50.
6. Yoon KJ, Oh BM, Kim DY. Functional improvement and neuroplastic effects of anodal transcranial direct current stimulation (tDCS) delivered 1 day vs. 1 week after cerebral ischemia in rats. Brain Research 2012; 1452: 61-72.
7. Bartha R, Drost DJ, Menon RS, Williamson PC. Spectroscopic lineshape correction by QUECC: combined QUALITY deconvolution and eddy current correction. Magn Reson Med 2000; 44(4): 641-5. 8. Bartha R, Drost DJ, Williamson PC. Factors affecting the quantification of short echo in-vivo 1H MR spectra: prior knowledge, peak elimination, and filtering. NMR Biomed 1999; 12(4): 205-16.
9. Clark VP, Coffman BA, Trumbo MC, Gasparovic C. Transcranial direct current stimulation (tDCS) produces localized and specific alterations in neurochemistry: a (1)H magnetic resonance spectroscopy study. Neurosci Lett 2011; 500(1): 67-71.
10. Hone-Blanchet A, Edden RA, Fecteau S. Online Effects of Transcranial Direct Current Stimulation in Real Time on Human Prefrontal and Striatal Metabolites. Biol Psychiatry 2016; 80(6): 432-8. 11. Rango M, Cogiamanian F, Marceglia S, et al. Myoinositol content in the human brain is modified by transcranial direct current stimulation in a matter of minutes: a 1H-MRS study. Magn Reson Med 2008; 60(4): 782-9.
Figures