2979
Posterior cingulate gyrus metabolism differs in parkinson’s disease patients with and without cognitive impairment1Department of Radiology, Affiliated Hospital of Guizhou Medical University, Guiyang, China, Guiyang, People's Republic of China
Synopsis
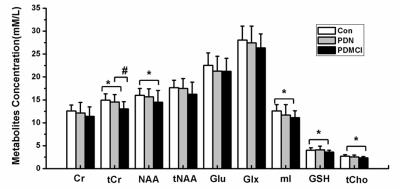
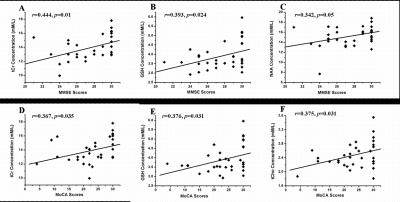
1H-MRS technology combined with Lcmodel software were used to quantitative the concentration of metabolites of posterior cingulate gyrus (PCC) in Parkinson’s disease patients with(PDCI) and without cognitive impairment(PDN), and MMSE and MOCA tests were also performed. Compared with controls, the concentration of total creatine (tCr), N-acetylaspartate(NAA), myo-Inositol(mI), Glutathione (GSH), as well as glycerophosphocholine + phosphocholine (tCho) were found significantly decreased in PCC in PDCI group , but no changes were found in PDN. Additionally, correlations between concentration of tCr, GSH, tCho, NAA and MMSE (MoCA) scores can also be found . These results may indicate that the metabolites abnormalities in PCC might be used as a biomarker to track cognitive decline in Parkinson's disease in a clinical setting.
Parkinson’s disease,1H-MRS, posterior cingulate gyrus, cognitive impairment
Introduction Most Parkinson's disease (PD) patients may develop cognitive impairment, and eventual dementia, and posterior cingulate gyrus (PCC) is highly involved in multiple cognitive processes. In this study, we were desired to find the concentration of metabolites of PCC in Parkinson’s disease patients with and without cognitive impairment (PDCI /PDN) and also to investigate the relationship between metabolites changes and cognitive status. So this study will be better to understand the potential role of MRS as in vivo molecular imaging biomarker for early diagnosis of PDCI and for monitoring the efficacy of therapeutic interventions.
Materials and methods 22 PDN patients, 12 PDCI patients and 22 age matched healthy older controls underwent PCC MRS. The MRS protocol was performed at 3 T using a Philips Achieva MRI system. Data were collected from a 2×2×2 cm voxel of interest (VOI) located in a portion of the PCC. Data acquisition used PRESS sequences with 2048 samples, spectral band width of 2000 Hz, and 128 acquisitions, using TR/TE = 2000/40 ms for total creatine (tCr), N-acetylaspartate (NAA), choline (Cho) and myo-Inositol (mI) (Fig.1), et al. Metabolites were quantified using LCModel.
Results Compared to controls, patients with PDCI showed significantly reduced tCr(p <0.01), NAA (p <0.05) , Cho (p <0.05) and mI (p <0.01)(Fig.2). But, no significantly metabolism changes were detect between patients with PDN and controls. Additionally, correlations between concentration of tCr(r=0.444, p=0.01), NAA(r=0.342, p=0.05), GSH(r=0.393, p=0.024) and Mini-Mental State Examination(MMSE) scores, and correlations between concentration of tCr(r=0.367, p=0.035), GSH(r=0.376, p=0.031), tCho(r=0.375, p=0.031) and Montreal Cognitive Assessment(MoCA) scores were found(Fig.3). Fig. 1 A: T1 sagittal MRI showing voxel of interest within the posterior cingulate gyrus, representative spectra from study participants: B) Healthy control participant (Con); C) patient with cognitive impairment(PDCI). NAA,N-acetylaspartate;tCho, glycerophosphocholine+phosphocholine; tCr, total creatine; mI, myo-Inositol; GSH, Glutathione; Glx, glutamate + glutamine.
Discussion The posterior cingulate cortex is a highly connected and metabolically active brain region, and it has an important cognitive role[1]. The potential role of MRS as in vivo molecular imaging biomarker to confirm early and differential PDCI diagnosis is controversial[2]. In this study, PDCI and PDN patients showed distinct PCC metabolic MRS profiles, compared with the controls, the concentration of tCr, NAA, mI, GSH, as well as tCho were found significantly decreased in PCC in PDCI group , but no changes were found in PDN, these results are consistent with many other studies[3-5]. The metabolic abnormalities in PCC maybe due to the lower regional cerebral blood flow in this region than normal controls[6]. Findings suggest that comparison of PCC MRS profiles across mild cognitive impairment provides useful information for tracking cognitive decline in Parkinson's disease in a clinical setting.
Acknowledgements
This work is supported by grants from the Natural Science Foundation of China (8156070059) and Science and Technology Department of Guizhou Province-Guizhou medical university, Qiankehe LG[2012]024.References
[1] Leech R, et al. Brain, 2014, 137(Pt 1): 12-32. [2] Ciurleo R, et al. Biomed Res Int, 2014, 2014: 519816. [3]Camicioli R M, et al. Neuroscience letters, 2004, 354(3): 177-180. [4] Sharma S, et al. Neurochemistry international, 2013, 63(3): 201-229. [5] Holmay M J, et al. Clinical neuropharmacology, 2013, 36(4): 103-106. [6] Kamagata K, et al. J Magn Reson Imaging, 2011, 33(4): 803-807.Figures