2973
Effect of psychotherapeutic approaches on cortical and striatal neurochemical profile in a juvenile rat model of ADHD: an in vivo 1H MRS study @ 11.7T1Core Facility Small Animal Imaging, Ulm University, Ulm, Germany, 2Institute of Anatomy and Cell Biology, Ulm University, Ulm, Germany, 3Department of Child and Adolescent Psychiatry, Ulm University, Ulm, Germany, 4Center for Magnetic Resonance Research, University of Minnesota, MN, United States
Synopsis
Proton MRS is employed to assess the effect of systemic administration of aripiprazole and riluzole on the neurochemical profile of distinct functional regions in juvenile rat brain non-invasively in vivo. In this study, a dedicated optimized STEAM sequence with single-shot phase and frequency correction, and image-based shimming was applied to quantify subtle changes in the brain metabolites concentration during drug treatment in an ADHD rat model.
PURPOSE
Attention-deficit/hyperactivity disorder (ADHD) is the most common psychiatric disorder (30-50%) during childhood (1). In 40-70% of childhood onset, the disorder continues during adolescence and in more than a half of the affected individuals ADHD persists into adulthood (2). The observation that the most effective drugs for ADHD treatment are psychostimulants (3), implicates a role for catecholamines in the development of the disease. In this study, a dedicated optimized STEAM sequence with single-shot phase and frequency correction, and image-based shimming was applied to quantify subtle changes in the brain metabolites concentration during drug treatment in an ADHD rat model.METHODS
In this gender- and age-matched vehicle-controlled designed study, each cohort of SHR rats underwent a subchronic pharmacological intervention with vehicle (saline containing 1% Tween 80, n=9), aripiprazole (1,5 mg/kg i.p., n=6) or riluzole (6 mg/kg i.p, n=5). The subchronic daily drug treatment was performed from PND 35 to PND 50. A home-built head restrainer was used to properly immobilize the animal's skull during measurements, ensuring stability and reproducibility of the experimental setup. In vivo 1H MR spectra were acquired from the striatum and prefrontal cortex on PND42. Experiments were performed at a dedicated small animal system (117/16 USR BioSpec, AVANCE III, ParaVision 6.01, Bruker BioSpin, Ettlingen, Germany) equipped with a 9 cm inner diameter self-shielded gradient coil insert providing 750 mT/m maximal strength in 80 μs rise time. A 72 mm birdcage quadrature volume resonator was used for excitation and a receive-only rat brain 2x2 element surface coil array was used for signal reception. Volume-of-interests (VOI) were planned based on T1-weighted 2D FLASH (TR/TE = 193/5ms, flip angle 17.5°) images. Field homogeneity was adjusted individually for each investigated region using a field-map based approach (MAPSHIM). A short-echo-time STEAM spectroscopy sequence (TR/TE/TM: 5000/3.5/10ms, 256 acquisitions) combined with VAPOR water suppression was used (4). Spectra were acquired from 18.5μl (striatum) and 27.5 μL (prefrontal cortex) volumes. Single-shot data were frequency and phase corrected prior to summation (5). Unsuppressed water signal was used as an internal reference as well as for eddy current correction and absolute metabolite concentrations were derived with LCModel (chemical shift range of 0.5–4.2 ppm) (6). Statistical significance of the differences was analyzed with the non-parametric Mann–Whitney U-test.Results and Discussion
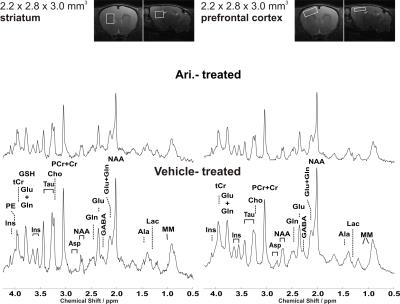
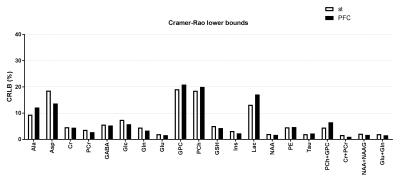
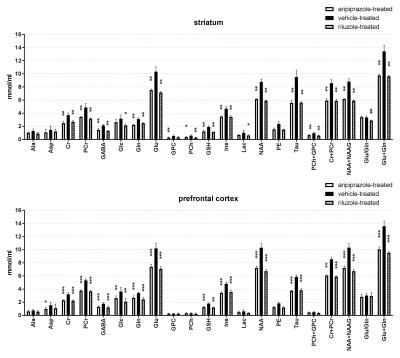
Representative water-suppressed in vivo proton MR spectra of the investigated brain regions for the different groups show obvious changes in the neurochemicals concentration (Fig. 1) for the drug treated group. The average full width at half-maximum found by LCModel was 0.023 ± 0.003 ppm (11.5 ± 1.5 Hz) in the striatum and 0.027 ± 0.003 ppm (13.5 ± 1.5Hz) in the prefrontal cortex of rats. Corresponding SNR were 23 ± 2 and 37 ± 5. The average Cramer-Rao lower bounds (CRLB) corresponding to the fitted metabolites are shown in Fig. 2. Average CRLB for GABA, Glu and Gln were 6, 2 and 5 in the prefrontal cortex and 5, 2 and 3 in the striatum, proving the reliability of the quantification of the metabolites of the glutamatergic and GABAergic neurotransmission systems. The high spectral quality achieved over the entire chemical shift range (0.5–4.2 ppm) ensured reliable and reproducible quantification of each of the brain metabolites. A detailed comparison of region-specific neurochemical profiles of aripiprazole- and riluzole-treated with vehicle-treated rats is shown in Fig. 3. In both brain regions, drug treatment led to significant reduction in most of the neurochemicals measured. Despite of the fact that scyllo-Ins and N-acetylaspartylglutamate (NAAG) signal were incorporated into the basis set of LCModel as a model component, corresponding concentration was not reliably possible. Conclusion: An optimized short TE STEAM sequences in combination with advanced single-shot frequency and phase correction, and image-based shimming enables the quantification of brain metabolites with high spectral fidelity and reproducibility, demonstrating altered neurochemical profiles of the aripiprazole- and riluzole-treated animals in comparison to vehicle-treated animals at very high magnetic field (11.75 T).Conclusion
An optimized short TE STEAM sequences in combination with advanced single-shot frequency and phase correction, and image-based shimming enables the quantification of brain metabolites with high spectral fidelity and reproducibility, demonstrating altered neurochemical profiles of the aripiprazole- and riluzole-treated animals in comparison to vehicle-treated animals at very high magnetic field (11.75 T).Acknowledgements
No acknowledgement found.References
1. Kessler RC, Adler L, Barkley R, Biederman J, Conners CK, Demler O, Faraone SV, Greenhill LL, Howes MJ, Secnik K, Spencer T, Ustun TB, Walters EE, Zaslavsky AM. The prevalence and correlates of adult ADHD in the United States: Results from the National Comorbidity Survey Replication. Am J Psychiatry. 2006 Apr;163(4):716-23.
2. Froehlich TE, Lanphear BP, Epstein JN, Barbaresi WJ, Katusic SK, Kahn RS. Prevalence, recognition, and treatment of attention-deficit/hyperactivity disorder in a national sample of US children. Arch Pediatr Adolesc Med. 2007 Sep;161(9):857-64.
3. Sagvolden T, Johansen EB, Aase H, Russell VA. A dynamic developmental theory of attention-deficit/hyperactivity disorder (ADHD) predominantly hyperactive/impulsive and combined subtypes. Behav Brain Sci. 2005 Jun;28(3):397-419; discussion 419-68.
4. Tkac I, Starcuk Z, Choi IY, Gruetter R. In vivo 1H NMR spectroscopy of rat brain at 1 ms echo time. Magn Reson Med (1999) 41:649.
5. http://www.cmrr.umn.edu/downloads/mrspa
6. Provencher, Automatic quantitation of localized in vivo 1H spectra with LCModel. NMR Biomed (2001) 14:260.
Figures