2968
Lactate is associated with tumour grading in breast cancer – An ex vivo study on whole breast tumours using multiple quantum coherence (MQC) MRS1Aberdeen Biomedical Imaging Centre, University of Aberdeen, Aberdeen, United Kingdom, 2Pathology Department, Aberdeen Royal Infirmary, Aberdeen, United Kingdom, 3School of Medicine, University of Aberdeen, Aberdeen, United Kingdom, 4Breast Unit, Aberdeen Royal Infirmary, Aberdeen, United Kingdom, 5Strathclyde Institute of Pharmacy and Biological Sciences, Glasgow, United Kingdom
Synopsis
Breast cancer is associated with increased lactate production in tumour, known as Warburg effect, that is postulated to enhance cancer cell survival advantage and invasiveness. However, current evidence, mainly based on xenografted animal models or biopsy results, remains controversial, where non-identical biological environments are compared to humans, or partial sampling error may play a role. We therefore examined the role of lactate concentration in whole human breast tumour, and hypothesised that there is a difference in lactate concentration between grade II and III breast cancer. To extract lactate under overwhelming lipid signal, MQC MRS was optimised for robust measurement.
Introduction:
Breast cancer is associated with tissue hypoxia and increased lactate production due to the upregulation of lactate dehydrogenase (LDH), known as Warburg effect1. It has been proposed that Warburg effect is related to stronger cancer cell survival advantage and invasiveness or metastatic risk. However, the current evidences remains controversial. Published literature knowledge is mainly based on xenografted (planted tumour) animal models or biopsy results2,3. Hence, these findings often suffer from non-identical biological environment compared to human or partial sampling error. We therefore set out to examine the role of lactate concentration in whole human breast tumour, and hypothesise that there is a difference in lactate concentration between grade II and III breast cancer.Methods:
Twenty-four female patients (12 grade II and 12 grade III) with invasive ductal carcinoma undergoing surgical intervention, were enrolled in the study. The patients have not received any hormonal treatment or chemotherapy. Upon the excision of the tumour, the fresh specimen was immediately transported to imaging facility as an acute study. To provide platform for in vivo translation and accommodate the physical dimension of the specimen, a whole body clinical scanner was chosen for this study. Once the scan was completed, the fresh tissue was transported to pathology for formalin treatment. The study was approved by NHS Research Ethics Service, and written informed consent was obtained prior to the MRS study.MRS Acquisition
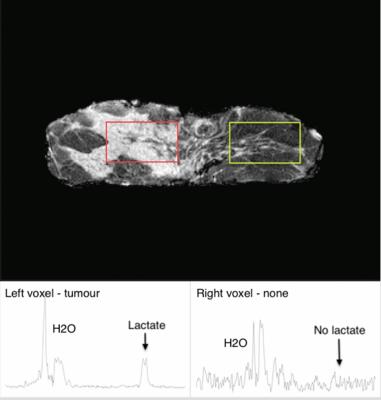
Spectra were acquired on a 3T whole body clinical MRI scanner (Achieva TX, Philips Healthcare, Best, Netherlands) using body coil for transmission and a 32-channel head coil as the receiver. Scan protocol contained standard high resolution T1 weighted anatomical images, unsuppressed water reference spectrum and lactate spectrum. Tumour was identified on the anatomical images by an experienced radiographer, while MRS voxel was positioned snug fit to the tumour boundary using PRESS localisation sequence. The unsuppressed water spectrum was collected with TR/TE 1.25s/144ms, 16 averages. Spectrum full width at half maximum (FWHM) was monitored (between 8 – 20Hz of all the samples) to ensure spectral quality. Lactate spectrum was obtained using MQC method4 implemented in pulse programming environment (PPE), with TR/TE 1.25s/144ms, spectral editing frequency at 4.1ppm, MQC encoding gradients of 50 and 100ms mT/m and 512 averages.Data Processing
Tumour volume and surface area were quantified by tracing the tumour boundary on anatomical images. Spectral data processing was performed in the jMRUI software. To allow internal referencing5, the unsuppressed spectra were processed using AMARES algorithm to derive water and lipid amplitudes. Lactate amplitude was derived from the MQC spectrum using AMARES algorithm, but a different set of prior knowledge. Absolute lactate concentration was subsequently obtained based on literature breast tissue water content level6 and relaxation properties7.Statistical Analysis
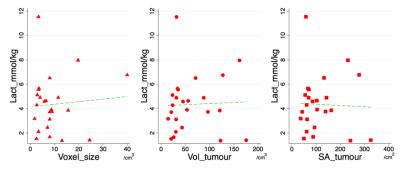
Statistical analysis was performed using the STATA software (StataCorp, College Station, Texas, USA). Two-sample t-test was carried out on the characterisation parameters to assess any bias between grade II and III tumour groups. Two-sample t-test was also performed on lactate concentration to examine its prognostic value. Correlation analysis was applied to determine the relationship between lactate concentration, voxel size, tumour volume and surface area. The statistical results were classified as significant if p value is smaller than 0.05.Results
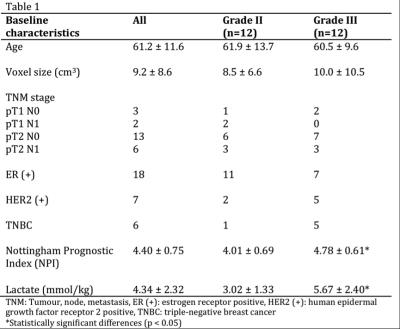
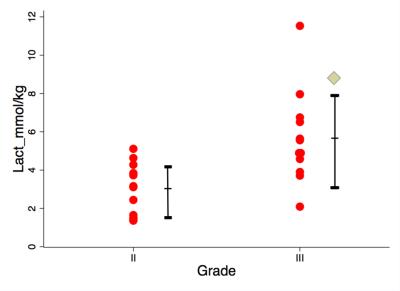
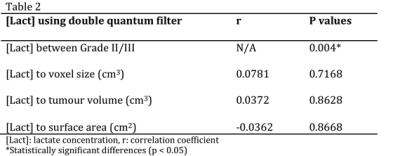
The sample characteristics of the two groups were shown in Table 1, where no statistically significant difference in age or voxel size was found. The mean tumour size was 2.6 ± 0.7 cm. The voxel positioning and spectra from the tumourous (and non-tumourous) regions for a specimen were shown in Figure 1, demonstrating the robustness of lactate measurement. For the entire cohort, the lactate concentration ranged from 1.4 to 11.5 mmol/kg. Lactate concentration was significantly higher in grade III samples compared to grade II (p=0.004, Figure 2, Table 2). The lactate concentration did not present significant correlation with voxel size, tumour volume or surface area (Figure 3, Table 2).Discussion
The lactate concentration obtained from MQC MRS is in agreement with literature findings using invasive methods, while allowing the observation of the whole tumour. MQC MRS provides superior lipid suppression capability, where no lipid leakage signal can be detected in a voxel situated away from the tumour in a mastectomy sample. The independence between lactate concentration and voxel size demonstrated the robustness of the methodology, while the independence from tumour volume and surface area confirms the measurement is targeting at specific processes fundamental to tumour metabolism.Conclusion
Lactate concentration is associated with breast tumour grading, confirming Warburg effects, and in turn providing a potential non-invasive prognostic marker via the application of MQC MRS.Acknowledgements
The authors would like to thank Dr Matthew Clemence
for clinical scientist support, Bolanle Awote for patient recruitment, Dawn Younie
for logistic support, Prof Andrew M Blamire for advice on MRS. This project is funded by
Friends of ANCHOR, and Sai Man Cheung is
jointly supported by Elphinstone scholarship, Roland Sutton Academic Trust and
John Mallard scholarship.
References
1. Gatenby, R. A., Gillies, R. J. Why do cancers have high aerobic glycolysis? Nature Reviews Cancer. 2004; 4(4): 891 – 899.
2. Magnitsky, S., Belka, G. K., Sterner, C., et al. Lactate detection in inducible and orthotopic Her2/neu mammary gland tumours in mouse models. NMR in biomedicine. 2013; 26(1): 35 – 42.
3. Rizwan, A., Serganova, I., Khanin, R., et al. Relationships between LDH-A, lactate, and metastases in 4T1 breast tumors. Clinical Cancer Research. 2013; 19(18): 5158 – 5169.
4. He, Q, Shungu, D.C., van Zijl, P.C.M., et al. Single-scan in vivo lactate editing with complete lipid and water suppression by selective multiple-quantum-coherence transfer (Sel-MQC) with application to tumors. Journal of Magnetic Resonance, Series B. 1995; 106(3): 203 – 211.
5. Bolan, P. J., Meisamy, S., Baker, E. H., et al. In vivo quantification of choline compounds in the breast with 1H MR spectroscopy. Magnetic Resonance in Medicine. 2003; 50(6): 1134 – 1143.
6. Sijens, P.E., Dorrius, M.D., Kappert, P., et al. Quantitative multivoxel proton chemical shift imaging of the breast. Magnetic Resonance Imaging. 2010; 28(3): 314 – 319.
7. Annarao, S., Thomas, K., Pillarsetty, N., et al. In vivo lactate T1 and T2 relaxation measurements in ER-positive breast tumours using SS-SelMQC editing sequence. Proceedings of the 19th Annual International Society of Magnetic Resonance in Medicine (ISMRM), Montréal, Québec. 2011; p.3158.
Figures