2967
7T MRSI Identifies Neuronal and Axonal Injury in a Limbic Network in MRI Negative Veterans with mTBI and PTSD1Radiology, University of Pittsburgh, Pittsburgh, PA, United States, 2Neurology, University of Pittsburgh, Pittsburgh, PA, United States, 3Neurology, Pittsburgh Veterans Administration Medical Center, PA, United States, 4Biostatistics, Pittsburgh Veterans Administration Medical Center, PA, United States
Synopsis
Following mild traumatic brain injury (mTBI) many veterans continue to experience persistent symptoms despite an absence of significant findings on conventional MRI. In this study we acquired MRSI and volumetric data from veterans with a history of mTBI and PTSD, healthy age matched controls and veterans with PTSD without a history of mTBI. Data was acquired from the hippocampus (single slice MRSI) and cingulate (multiband MRSI). Significant declines in NAA/Ch were seen in the hippocampal formation in comparison to healthy controls and veterans with PTSD. Further, reductions in hippocampal NAA/Ch were statistically correlated with reductions in NAA/Ch from the cingulate
Introduction
Following mild traumatic brain injury (mTBI) many veterans continue to experience persistent symptoms despite an absence of significant findings on conventional MRI. Further complicating their care, many of these veterans also have a diagnosis of post-traumatic stress disorder (PTSD). Given the similarity of clinical symptoms between mTBI and PTSD this leaves little objective information upon which to guide and differentiate their treatment. Thus an objective assessment that can: 1) identify quantitative measures of injury and 2) potentially resolve the effects of mTBI and PTSD, would be of significant utility. Previously we evaluated veterans with ongoing self-reported memory deficits with a history of blast related mTBI demonstrating significant alterations in the hippocampi in comparison to healthy controls (1,2). In this study we broadened the population to include veterans with mTBI and PTSD with or without ongoing self- reported memory impairments.Methods
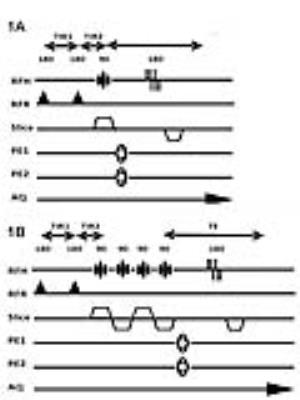
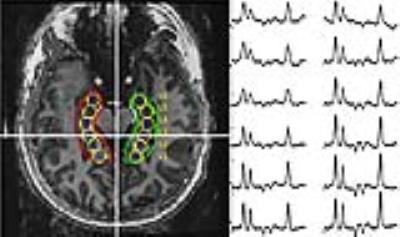
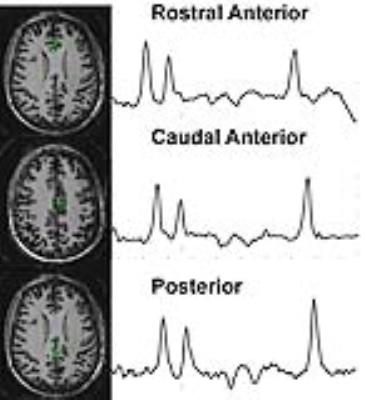
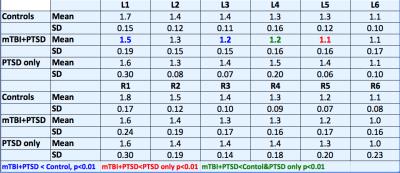
To evaluate these two questions we acquired MRSI and volumetric data from veterans with a history of mTBI and PTSD (mtBI+PTSD, n=38), healthy age matched controls (n=15) and veterans with PTSD without a history of mTBI (PTSD only, n=5). In keeping with previous work we acquired single slice data angulated along the hippocampal plane. To evaluate the extent to which the hippocampal injury reflects a limbic network of injury, we also acquired 4-slice multiband MRSI data (3) to evaluate the cingulate in n=14 veterans with mTBI and PTSD. All data were collected at 7T using a 16 channel (8x2, columns x rows) transceiver array (4) and a high order/degree shim insert (5). MRSI data were acquired using a slice selective spin echo sequence with TE/TR of 40/1500 with 24x24 encodes across a FOV of 240x240mm. B1 shimming based outer volume suppression was used to suppress extra-cranial tissues (6). Water suppression was provided by a semi-selective refocusing pulse and a frequency selective inversion recovery (Fig 1a). For the multi-band acquisition (Fig 1b), the single slice selective excitation pulse was replaced with four cascaded slice selective pulses and the phase of these pulses was toggled depending on the encoding step (kx, ky) to reduce spatial overlap (3). To account for tissue heterogeneity, the hippocampus was divided into six loci (Fig 2) and the cingulate was divided into 3 parcels posterior, caudal anterior and rostral anterior (Fig 3). An MP2RAGE sequence (7), 0.7mm isotropic resolution, was used for both volumetric analysis of the hippocampi and identification of cingulate parcels with Freesurfer.Results
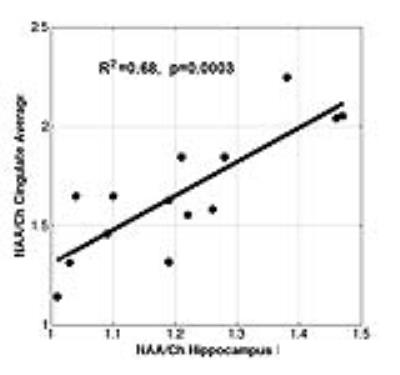
Displayed in Table 1 are summary data from the hippocampus. In comparison to controls, after bonferoni correction, NAA/Choline (NAA/Ch) was significantly reduced in loci 1,3 and 5 (p<0.01). Although the mTBI+PTSD and PTSD only groups were indistinguishable in terms of depression, anxiety and severity of PTSD, significant declines in NAA/Ch were seen in the left hippocampus (L4 and L5). Volumetric analysis of the hippocampi showed no significant difference in volume between the three groups. The number of mTBIs in the mTBI+PTSD group was associated with decreased NAA/Ch. To explore the extent to which these significant reductions in NAA/Ch are propagated through a limbic network including the hippocampus and cingulate we correlated NAA/Ch levels in the hippocampus and cingulate. Statistically significant correlations were seen with the average of L2-5,R2-5 of the hippocampus with all three cingulate loci individually (R=0.40-0.61, p0.02-0.0009,) and their combined average total pool (R2=0.68,p=0.0003), Fig 4.Discussion
Similar to previous work significant declines in NAA/Ch were seen in the hippocampal formation in comparison to healthy controls. This suggests that the injury to the hippocampus is not specific to veterans with ongoing memory impairment, but potentially reflective of selective vulnerability of the hippocampus. Although the PTSD population is small (n=5), in comparison, the mTBI+PTSD group shows significant declines in the anterior hippocampus. This suggests that decline in NAA/Ch seen in the hippocampus is not solely due to PTSD, but rather reflects mTBI or the synergistic effects of mTBI and PTSD. The absence of significant reductions in hippocampal volume suggests that neuronal loss is not driving the changes seen and the damage may still be reversible. The severity of reductions in hippocampal NAA/Ch were also highly correlated with reduced NAA/Ch across the cingulate suggesting a mechanism whereby dysfunction and injury propagates along a limbic network. These findings may provide both a basis and rationale for tailoring the clinical care of this group. Since this study represents a cross sectional evaluation, the long term progression or absence of progression of the damage is unknown.Conclusions
MRSI of the hippocampus and cingulate provides a sensitive measure for detecting injury in veterans with mTBI and PTSD.
Acknowledgements
NIH: R01 NS090417, R01 NS081772, R01 EB009871References
1. de Lanerolle NC, Hamid H, Kulas J, Pan JW, Czlapinski R, Rinaldi A, Ling G, Bandak FA, Hetherington HP. Concussive brain injury from explosive blast. Ann Clin Transl Neurol 2014;1(9):692-702. 2. Hetherington HP, Hamid H, Kulas J, Ling G, Bandak F, de Lanerolle NC, Pan JW. MRSI of the medial temporal lobe at 7 T in explosive blast mild traumatic brain injury. Magnetic resonance in medicine 2014;71(4):1358-1367. 3. Hetherington H, Zhao T, Yushmanov V, Pan J. Multi-Band MRSI at 7T using 3D B1 Shimming based Outer Volume Suppression Society for Magnetic Resonance in Medicine. Singapore2016. p 378. 4. Avdievich NI. Transceiver-Phased Arrays for Human Brain Studies at 7 T. Applied magnetic resonance 2011;41(2-4):483-506. 5. Pan JW, Lo KM, Hetherington HP. Role of very high order and degree B0 shimming for spectroscopic imaging of the human brain at 7 tesla. Magnetic resonance in medicine 2012;68(4):1007-1017. 6. Hetherington HP, Avdievich NI, Kuznetsov AM, Pan JW. RF shimming for spectroscopic localization in the human brain at 7 T. Magnetic resonance in medicine 2010;63(1):9-19. 7. Marques JP, Kober T, Krueger G, van der Zwaag W, Van de Moortele PF, Gruetter R. MP2RAGE, a self bias-field corrected sequence for improved segmentation and T1-mapping at high field. NeuroImage 2010;49(2):1271-1281.Figures