2964
Sodium MRI of the thyroid gland at 7 tesla1Imaging Division, University Medical Center, Utrecht, Netherlands, 2Medical Physics in Radiology, German Cancer Research Center (DKFZ), Heidelberg, Germany, 3Institute of Radiology, University Hospital Erlangen, Erlangen, Germany, 4Biomedical Image Analysis, Eindhoven University of Technology, Eindhoven, Netherlands
Synopsis
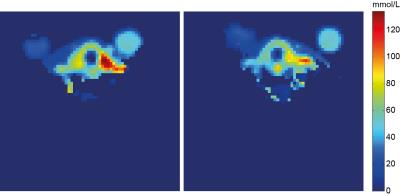
This work shows the potential of sodium imaging of the thyroid gland in vivo at 4mm isotropic resolution integrated to 1H imaging. An optimized setup combined with tuned sequences and B1 corrections enables quantitative sodium mapping of the thyroid gland and its surrounding tissue. The thyroid gland has the highest concentration of sodium in this part of the neck, estimated to be 64.5 mmol/L in vivo. Sodium imaging might open up the detection of (lymph node) metastases of thyroid cancer, as they are expected to exceed the healthy concentration of sodium detected in the head and neck region.
Purpose
To investigate feasibility of sodium imaging of the thyroid gland in healthy volunteers.Introduction
The thyroid gland takes up iodine via the sodium/iodide symporter. The activity of this symporter is affected in multiple thyroid diseases, including thyroid cancer, and leads to modifications in sodium concentrations 1. Nowadays, diagnostic imaging of the thyroid is performed using ultrasonography and/or scintigraphy using radioisotopes 2. Using MRI, extracapsular extension, esophageal and tracheal invasion might be better detected 3-5. We hypothesize that (23Na and 1H) MRI gives more specific anatomical and physiological information that will be beneficial for disease staging and treatment choice provided that the sensitivity of sodium in MRI is sufficient. As a first step we here report on the feasibility of 1H and 23Na MRI of the thyroid gland in healthy subjects using a high sensitivity sodium setup at 7T with fully decoupled dipole antennas for 1H imaging. Furthermore, the usage of a B1 correction technique to obtain quantitative sodium maps has been investigated using another setup.Methods
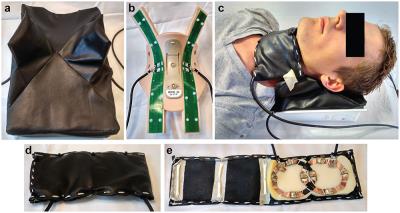
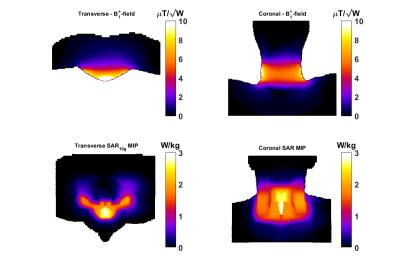
A 23Na/1H coil array setup has been realized for thyroid imaging at 7T consisting of two posterior fractionated dipole antennas6 for 1H and an anterior quadrature loop pair for 23Na, Figure 1. The 1H antennas are inherently decoupled, the 23Na coils are decoupled by overlap. For the 1H antennas the average power limit of 3W per channel is assumed, derived from body imaging applications using 8 channels; the 23Na coils have been simulated with Sim4Life (ZMT, Zurich, CH) to assess the SAR10g distribution, Figure 2, resulting in a power limit of 6W for both channels combined. 5 volunteers were scanned using a 7T MR scanner (Philips, Best, Netherlands). Proton anatomical imaging was obtained with a 3D FFE sequence (TR/TE=4.0/1.9ms, acquired voxel size=1x1x1mm3, FOV=200x325x100mm3). Sodium imaging was acquired using the following parameters TR/TE=10/1.9ms, acquired voxel size=4x4x4mm3, nominal FA=4.4 . To demonstrate the potential of using an optimized 23Na sequence including B1 correction the remaining two volunteers were scanned with another 7T scanner (Magnetom 7T, Siemens Healthcare, Erlangen, Germany). A double tuned (23NA/1H) flex surface coil (Rapid Biomedical, Rimpar, Germany) was used. For this setup, a density adapted 3D radial pulse sequence7 was used for 23Na MRI: TE = 0.35ms, TR = 160 ms, FA = 90°, NProjections = 4000, acquired voxel size = 3x3x3mm3, acquisition time TAQ = 10:40min. B1 maps8,9 were calculated using two 3D 23Na data sets acquired with preparation pulse phases ϕ = 0° and ϕ = 180° and used for B1 corrections. The data were reconstructed using a Nonuniform Fast Fourier Transform (NUFFT)10. To obtain quantitative measures, two phantoms containing a 0.15% (25.57mM) and 0.3% (51.3mM) sodium concentration were placed close to the thyroid gland.
Results
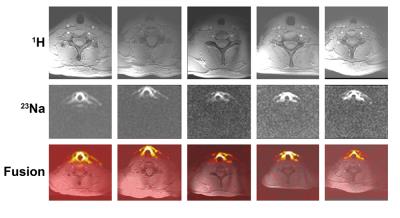
The in-house built thyroid gland setup shows a full coverage of the thyroid gland for both 1H and 23Na imaging, Figure 3. The antennas located at the posterior part of the neck ensure decoupling to the 23Na coils and a full 1H coverage of the neck, making it possible to not only visualize the thyroid, but also the surrounding tissue like: muscles, blood vessels, lymph nodes, the trachea and esophagus, important regions to visualize for disease staging. In the sodium image the thyroid can be clearly detected, furthermore the muscles and vessels are distinguishable. The use of an optimized 23Na sequence with B1 correction results in a quantitative measurement of the sodium concentration, Figure 4. As can be noticed, the thyroid gland has the highest concentration of sodium in this part of the neck, estimated to be 64.5 mM, Figure 5.Discussion & Conclusion
This work shows the potential of sodium imaging of the thyroid gland in vivo at 4mm isotropic resolution integrated to 1H imaging. An optimized setup combined with tuned sequences and B1 corrections enables quantitative sodium mapping of the thyroid gland and its surrounding tissue. Sodium imaging might open up the detection of (lymph node) metastases in the head and neck region, as metastases are expected to exceed the healthy concentration of sodium detected in the region. Furthermore, improved 1H anatomical imaging can show invasion into the trachea and esophagus. Both metastases and extracapsular invasion are up to now difficult to visualize using conventional sonography and scintigraphy. For other thyroid diseases, such as thyroiditis, hypothyroidism and hyperthyroidism, sodium imaging might be applied for follow up during treatment.Acknowledgements
This project has been supported by the Foundation "De Drie Lichten" (The Netherlands) and by the Bayer Science & Education Foundation (Germany) through a Carl Duisberg fellowship.References
1 Carvalho et al. Arq Bras Endocrinol Metabol 2007
2 King et al. Clin Radiol 2000
3 Noda et al. AJR Am J Roentgenol 2015
4 Lu et al. Thyroid 2015
5 Shi et al. J Magn Reson Imaging 2016
6 Raaijmakers et al. Magn Reson Med 2015
7 Nagel et al. Magn Reson Med 2009
8 Maier et al. Magn Reson Med 2015
9 Allen et al. Magn Reson Med 2011
10 Fessler et al. IEEE Trans. Signal Process. 2003
Figures