2955
Direct Partial Volume Corrected CMRO2 Determination: Simulation assisted Dynamic 17O-MRI1Medical Physics in Radiology, German Cancer Research Center (DKFZ), Heidelberg, Germany, 2Institute of Radiology, University Hospital Erlangen, Erlangen, Germany
Synopsis
A dynamic 17O-MRI inhalation experiment enables localized mapping of the cerebral metabolic rate of oxygen consumption (CMRO2) in the human brain via H217O quantification. These functional information are tissue viability parameters and can help studying the brain metabolism. In 17O-MRI, accurate quantification and CMRO2-determination is severely biased by partial volume effects caused by low spatial resolution and fast transverse relaxation. A human brain-simulation providing realistic dynamic 17O-data was used to evaluate the performance of a partial volume correction algorithm at different temporal resolution. Findings were then adapted to an in-vivo 17O-MRI inhalation experiment which was conducted in a healthy volunteer.
PURPOSE
The oxygen metabolism (oxidative phosphorylation) in the human brain is an indicator of cell viability. The only stable MR-visible oxygen isotope (17O, nat. abundance 0.037%) can be utilized in a dynamic inhalation experiment for a localized determination of the cerebral metabolic rate of oxygen consumption (CMRO2) by quantification of H217O[1]. A changing CMRO2-value can for example be seen in tumor cells[2] (‘Warburg Effect’) or in Alzheimer’s disease[3]. Therefore, an accurate and localized CMRO2-determination is of interest to study the metabolism under various conditions or for treatment evaluation. Quantification accuracy of 17O-MRI is severely limited by partial volume (PV) effects, that are caused by fast T2*-relaxation and low spatial resolution of (5-10mm)3. Latter is caused by a factor of 106 reduced in-vivo 17O-signal compared to protons. Therefore, pulse sequences that enable ultra-short echo-times and high SNR-efficiency such as 3D-density-adapted radial (3D-DAPR)[4] or twisted-projection imaging[5] are used. For quantification in non-proton MRI, a partial volume correction (PVC) algorithm[6] was already successfully applied[7,8]. In the presented study a dynamic 17O-inhalation experiment of a healthy volunteer was conducted. Effects of temporal resolution, a sliding-window-reconstruction technique and PVC were evaluated by simulations and findings were applied to experimental data.METHODS
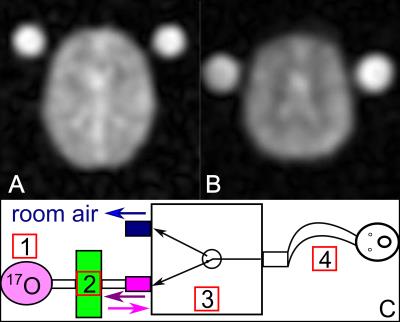
An in-house developed human brain-simulation was modified for simulation of dynamic signal evolution analogous to an inhalation experiment (Fig.1A). Tissue water-concentration[9] (cW) was simulated as follows: GM-cW =80% and CMRO2=2.0μmol/g*min; WM-cW=69% and CMRO2=0.7μmol/g*min and CSF-cW =100% (no metabolism). Dynamic 17O-data was reconstructed with variable temporal resolution Δt (Δt=0:30min-2:00min) and PVC was applied to every data-set for quantification of H217O. Three compartments (CSF, grey (GM) and white matter (WM)) were considered and T2*-decay[10] was incorporated into simulation and correction. A three-phase metabolic model[1] was fitted to corrected data to obtain CMRO2-values in considered brain compartments.
For the in-vivo experiment, a MR-compatible breathing-system, administering a variable 17O-bolus in a closed circuit (Fig.1C), was used. Imaging was conducted with a custom-built 17O/1H-head-coil on a 7T MR-system[11]. 17O-data was acquired with a 3D-DAPR-sequence using a Golden-Angle (GA) projection acquisition-scheme[12] allowing variable Δt with a nominal spatial resolution of (7.5mm)3 (Fig.1B). During the three-phase-experiment (TAcq=40:00min), 4.0±0.1L of 70%-enriched 17O-gas[13] was inhaled by a male volunteer (age 65): baseline-phase (10:00min, room-air), 17O-inhalation phase (11:30min), decay phase (17:30min, room-air). Additional data for B1-correction[14] and anatomical 1H-data were acquired as registration- and segmentation-basis. Post-processing of measured data-sets was conducted analogous to simulations.
RESULTS
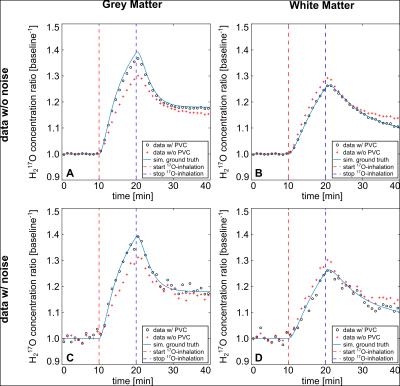
Quantification results were verified with the simulation’s ground-truth (GT): PV-corrected data, with and without simulated noise showed deviation of cW of 1-8% for GM and 0-5% for WM. In contrast, 25-32% for GM and 11-16% for WM was observed for non-corrected data, respectively. PV-bias was also seen for CMRO2-values determined before PVC. The PVC showed improvement with minor influence on chosen Δt (Tab.1/Fig.2) for GM and WM.
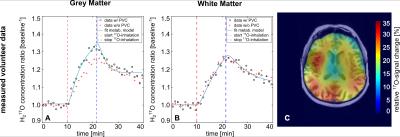
Obtained cW-values for PV-corrected experimental data in the baseline-phase was within ±5% of expected value[9] for all considered compartments. The enrichment-factor α of administered 17O-gas in the breathing-system was estimated to 49±3% and determined CMRO2-values were: CMRO2=2.07±0.15μmol/g*min (GM) and CMRO2=0.65±0.03μmol/g*min (WM) (Tab.2/Fig.3). A sliding-window-reconstruction (SWR) where consecutive reconstruction time-frames were shifted backward by Δt/2 was also applied, evaluated in simulations and adopted to in-vivo data (Tab.1B/Tab.2), showing no change in obtained CMRO2.
DISCUSSION
Utilization of the brain-simulation allowed direct verification of dynamic 17O-data analysis in a realistic setting: strong PV-influence was seen for H217O-concentration and CMRO2-values which led to over- and underestimation of the metabolic rate (19-55%). The applied PVC is able to correct CMRO2-values close to the GT (max. deviation 8.5%) by decreasing the PV-bias. The GA-acquisition allowed evaluation of variable Δt and the SWR: CMRO2-values showed negligible influence on chosen Δt and SWR, whereas application of a SWR reduced fitting errors. Inaccuracies in quantification and fitting uncertainty are mainly influenced by decreasing SNR with lower Δt.
The in-vivo data exhibited similar behavior for varying Δt and SWR. WM CMRO2-values (lowest expected PV-bias) are in good agreement with other studies[1,7,15,16] (Tab.2) whereas GM CMRO2-values show more variation: studies without PVC state an up to 30% lower GM CMRO2-value. However, in-vivo GM and WM baseline water-concentration is still underestimated by 4-6%, most likely due to not fully corrected transverse relaxation which can also slightly influence determined CMRO2-values.
CONCLUSION
The presented simulation-assisted dynamic 17O-MRI experiment verifies the applied PVC-algorithm, improves CMRO2-determination and optimizes post-processing. The ability to investigate Δt and applied SWR helps to save expensive 17O-gas. This approach will be pursued in further inhalation measurements aiming to show reproducibility in volunteer studies. Furthermore, our breathing system has a low breathing resistance allowing patient measurements.Acknowledgements
No acknowledgement found.References
[1] Atkinson et al., Neuroimage 2010 (51): 723–733, [2] Miles KA, Williams RE., Cancer Imaging 2008 (8):81-86, [3] Beal MF., Ann Neurol; 1992 (2):119-130, [4] Nagel et al., Magn Reson Med 2009 (62):1565-73, [5] Boada et al., Magn Reson Med (1997); 37: p. 706-715, [6] Rousset et al., J Nucl Med 1998(5):904-911, 1998, [7] Hoffmann et al., MAGMA 2014(27):579-87, [8] Niesporek et al., NeuroImage 2015(112): 353–363, [9] Neeb et al., NeuroImage 2006 (31): 1156–1168, [10] Niesporek et al., Proc. ISMRM 24 (2016, #3964), [11] Magnetom 7T, Siemens AG, Erlangen, Germany [12] Chan, R.W. et al., Magn Reson Med 2009(61): p. 354–363, [13] NUKEM Isotopes Imaging GmbH, Alzenau, Germany, [14] Morell, Magn Reson Med 2008 (60):889-94, [15] Hoffmann et al., Magn Reson Med; 66:1109-15 (2011), [16] Borowiak et al., Proc. ISMRM 23 (2015, #4633)Figures