2953
Measurement of CMRO2 in conscious rat with in vivo 17O MRS at 16.4T1CMRR, Radiology, University of Minnesota Medical School, Minneapolis, MN, United States, 2Department of Biomedical Engineering, Pennsylvania State University, University Park, PA, United States
Synopsis
In this study we performed dynamic in vivo 17O MRS measurements in conscious and isoflurane-anesthetized rat during inhalations of 17O2-enriched gas at ultra-high field of 16.4T, ultimately to compare the relation between CMRO2 and brain condition.
Introduction
The cerebral metabolic rate of oxygen (CMRO2) is an important physiological parameter reflecting the state of the brain tissue in its ability to consume oxygen and produce ATP energy. It is well controlled by the physiological auto-regulation in conscious state, and is highly sensitive to anesthetics and external environment when under anesthesia condition. The measurement of CMRO2 in awake and spontaneously breathing animal is difficult although it could provide an even closer representation of the metabolic state of the human brain.
In this study we performed dynamic in vivo 17O MRS measurements in conscious and isoflurane-anesthetized rat during the inhalations of 17O2-enriched gas at ultra-high field of 16.4T, ultimately to compare the relation between CMRO2 and brain condition.
Materials and Methods
Animal training and physiological monitoring
The MR scan protocol for performing conscious animal studies is challenging and requires special setup and training, which was adapted from resting-state fMRI studies under awake condition 1,2. A female Sprague-Dawley rat (~270g) was trained over 4 weeks with a conditioning protocol and a custom-built 3D-printed restraint device designed specifically for leakage free application of breathing gas. A mock MRI, i.e., a dark and noise-insulated tube was used for the training during which the scanner sound was played to the animal 1,2. Special care was taken to match both the sound level and frequencies of recorded MR-sequences (~ 131 dB - 135 dB). A pressure-sensor was placed at the animal chest to measure the respiration motion and the body temperature was monitored with a fiber optic probe. The animal reached a trained state and was transitioned to magnet habituation after 3 weeks since it showed a resting respiration rate of ~80-100 per minutes with significant reduction of motion in the restraint holder. Isoflurane anesthesia was used for induction. In the final week, a daily training with inhalation periods of 3-6 min during awake resembling the 17O2 tracer administration was performed. Protocols were approved from the IACUC of the University of Minnesota.
MRI setup
1H MRI and 17O MRS acquisitions were performed at 16.4T using custom-built loop surface coils tuned to a single channel 1H and quadrature 17O frequency under 2% isoflurane anesthesia and conscious awake conditions. Measurements of 17O-CMRO2 were performed with surface-coil localized FID (TR 11ms, nt=182 each 2s) and/or 3D-CSI (TR 11ms, TE 0.45ms, 3D volumes in 11.3s each, axial orientation, BW 30kHz, FOV 4x4x4 cm3) acquisitions. First, two scans were performed during Isoflurane 2% and after awakening the remaining scan time was one hour for a repeat of the previous scans. Time courses were normalized to natural abundance of H217O (0.037%) and fitted with a three phase model for metabolic turnover (alpha 17O2 74.8%) and circulation parameters3.
Results
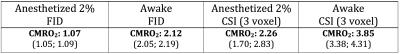
Respiration rates during awake conditions were within limits of natural fluctuations observed during training. Fit quality was slightly reduced in the awake condition due to motion-induced irregularities, but still produced highly significant estimations for contrasting metabolic rates between the two conditions (p<0.05): e.g., about 70-100% higher value of CMRO2 in the awake condition measured by either global brain FID or 3D-CSI (see Figure 1 and Table 1). The CMRO2 values measured by global FIDs are significantly lower than that of 3D-CSI and the washout periods of the FID data also showed a relatively slower exponential decay 4 in both anesthetized and awake conditions because the 17O RF coil partially covered the surrounding muscle tissue with much lower perfusion as compared to the brain tissues (i.e., partial volume effect).Discussion and Conclusion
We have performed the first metabolic measurement in an awake and trained rat during 17O2 gas inhalations at 16.4T. The difference in CMRO2 measurements between the 2% isoflurane anesthesia and the awake condition was high (almost doubled in awake rat). The current study demonstrated the feasibility of conducting metabolic imaging in conscious animal, although it could be more challenging than awake fMRI study when relatively long acquisition times are taken into account.Acknowledgements
NIH Grants: RO1 NS70839, MH111447; R24 MH106049, P41 EB015894, P30 NS076408 and S10 RR025031 entitled “Ultra-High Field (16.4 Tesla) Animal MRI/MRS System”.References
1. King, J. A. et al. Procedure for minimizing stress for fMRI studies in conscious rats. J. Neurosci. Methods 148, 154–160 (2005).
2. Liang, Z., King, J. & Zhang, N. Uncovering Intrinsic Connectional Architecture of Functional Networks in Awake Rat Brain. J. Neurosci. 31, 3776–3783 (2011).
3. Atkinson, I. C. & Thulborn, K. R. Feasibility of mapping the tissue mass corrected bioscale of cerebral metabolic rate of oxygen consumption using 17-oxygen and 23-sodium MR imaging in a human brain at 9.4 T. NeuroImage 51, 723–733 (2010).
4. Zhu, X.-H., Zhang, Y., Wiesner, H. M., Ugurbil, K. & Chen, W. In vivo measurement of CBF using 17O NMR signal of metabolically produced H217O as a perfusion tracer. Magn. Reson. Med. (2012). doi:10.1002/mrm.24469
Figures