2952
Dynamic Oxygen-17 MR Imaging with Golden-Ratio-Based Radial Sampling and k-Space-Weighted Image Reconstruction1Department of Biomedical Engineering, Case Western Reserve University, Cleveland, OH, United States, 2Case Center for Imaging Research, Case Western Reserve University, Cleveland, OH, United States, 3Department of Chemistry, Case Western Reserve University, Cleveland, OH, United States, 4Department of Radiology, Case Western Reserve University, Cleveland, OH, United States, 5Department of Physiology and Biophysics, Case Western Reserve University, Cleveland, OH, United States
Synopsis
This study aimed at developing a 3D dynamic oxygen-17 (17O) MR imaging method to delineate the kinetics of 17O-water uptake and washout in mouse brain with glioblastoma at high temporal and spatial resolution. A 3D imaging method using a stack-of-stars golden-ratio-based radial sampling scheme was combined with k-space weighted image reconstruction to improve the temporal resolution with preserved spatial resolution. The proposed method achieved a temporal resolution of 7.56 s with a voxel size of 5.625 μL in mouse brain at 9.4T. It can also be used to image cerebral oxygen consumption rate in 17O inhalation studies.
Purpose
Detection of oxygen in vivo is of great value in tracing water dynamics and/or quantification of oxygen metabolism, which plays an important role in disease diagnosis and treatment evaluation1. 17O is the only stable oxygen isotope that is MR detectable, it can thus serve as a tracer for water or oxygen. However, signal-to-noise ratio (SNR) of 17O MRI is extremely limited due to its low natural abundance and low MR sensitivity. The current study aimed at developing a three-dimensional (3D) dynamic 17O MR imaging method to delineate the kinetics of 17O-water uptake and washout in mouse brain with glioblastoma (GBM) at high temporal and spatial resolution.Methods
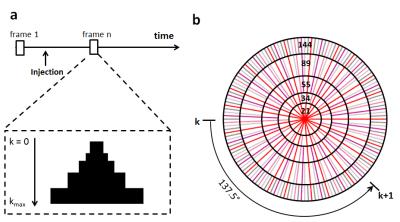
A 3D imaging method with a stack-of-stars golden-ratio-based radial sampling scheme was employed to acquire 17O signal. This sampling scheme achieves a nearly uniform coverage of k-space with an arbitrary number of spokes, and k-space coverage becomes most uniform when the number of spokes reaches a Fibonacci number2. Dynamic golden-angle acquisition was combined with k-space weighted image contrast (KWIC) reconstruction to achieve high temporal resolution with preserved spatial resolution3. To reconstruct a specific time frame, the k-space was segmented into a set of rings (Fig. 1). The central ring consisted of radial spokes acquired at the time frame of interest, with the number of spokes being a Fibonacci number (e.g., 21). Moving out in k-space to the next ring, the number of spokes increased to the next Fibonacci number. The radius of each ring was chosen such that the number of spokes in each ring fulfilled the Nyquist criterion3. After the k-space filtering, all images were reconstructed using non-uniform fast Fourier transform (NUFFT) toolbox4 with density compensation5.
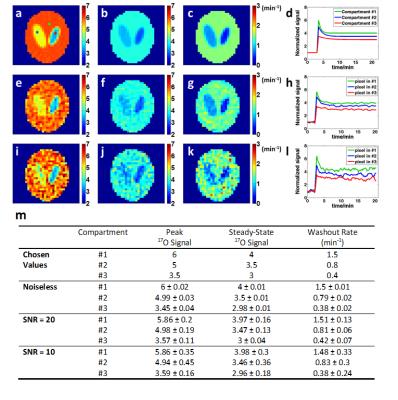
Simulation studies at different noise levels were performed on a modified Shepp-Logan phantom with three compartments with varied 17O signal kinetics. 10 athymic NCR nu/nu male mice were injected with GBM cells at 6-8 weeks of age and scanned on a Bruker 9.4T scanner at the age of ~36 weeks. 5 control mice were scanned at ~11 weeks. An in-house built, 2.5-cm diameter 17O surface coil was placed on top of the mouse head for 17O image acquisition. Seven fully sampled data sets were acquired at baseline before an intravenous bolus injection of 17O-water (200 μL, 13% enrichment). Data acquisition lasted for ~22 min during and after the injection. Imaging parameters were: TR/TE, 9 ms/0.367 ms; FOV, 4.8×4.8×1.25 cm3; matrix size, 32×32×5; number of averages, 8. These parameters yielded a voxel size of 5.625 μL. During reconstruction, the k-space filter was shifted by 21 spokes from one time frame to the next, leading to a temporal resolution of 7.56 s.
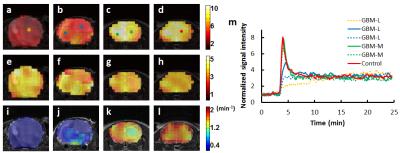
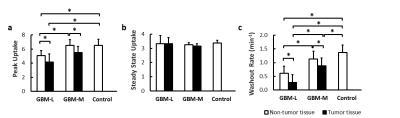
ROIs containing tumor and normal tissues were drawn manually from T2-weighted images acquired using rapid acquisition with relaxation enhancement (RARE) sequence. The percentage of tumor volume was calculated as the tumor volume divided by total brain volume. Mice with >30% tumor volume were classified as the GBM-Large (GBM-L) group, and mice with <30% tumor volume were designated as the GBM-Moderate (GBM-M) group. Maps of peak and steady-state 17O-water uptake and washout rate were generated by pixel-wise fitting of the 17O signal during the washout phase to a mono-exponential function. Mean values of these parameters were calculated from ROIs containing tumor and normal tissue, respectively.
Results
Simulation studies showed increased variations of the estimated parameters with decreased SNR. However, mean values of the parameters suggested unbiased estimation at different noise levels (Fig. 2). Post-injection 17O signal followed an exponential decay with a washout rate of ~1.36 min-1 in the brain of the control mice. GBM mice showed more heterogeneous 17O signal kinetics. In GBM-M group, 17O signal kinetics in brain tissue was the same as the controls (Fig. 3). However, tumor tissue exhibited prolonged washout (Fig. 4). In GBM-L group, tumor tissue showed no obvious washout phase, or even continuous accumulation of 17O-water after the injection (Fig. 3). In addition, peak 17O-water uptake was also significantly reduced in brain tissue, with prolonged washout (Fig. 4).Discussion & Conclusion
The proposed 17O imaging method achieved a temporal resolution of 7.56 s with a voxel size of 5.625 μL. Reduced uptake and prolonged washout of 17O-water were observed in tumor, suggesting compromised cerebral perfusion. Cerebral perfusion to normal brain tissue was also reduced in GBM-L mice. This study demonstrated a promising dynamic 17O imaging approach capable of delineating 17O-water kinetics in vivo with high temporal and spatial resolution. It can also be used to image cerebral oxygen consumption rate in 17O inhalation studies.Acknowledgements
No acknowledgement found.References
1. Zhu X-H, Chen JM, Tu T-W, Chen W, Song S-K. Simultaneous and noninvasive imaging of cerebral oxygen metabolic rate, blood flow and oxygen extraction fraction in stroke mice. Neuroimage [Internet] 2013;64:437–447. doi: 10.1016/j.neuroimage.2012.09.028.
2. Winkelmann S, Schaeffter T, Koehler T, Eggers H, Doessel O. An optimal radial profile order based on the golden ratio for time-resolved MRI. IEEE Trans. Med. Imaging 2007;26:68–76.
3. Song HK, Dougherty L. k-Space weighted image contrast (KWIC) for contrast manipulation in projection reconstruction MRI. Magn. Reson. Med. 2000;44:825–832.
4. Fessler JA, Sutton BP. Nonuniform fast Fourier transforms using min-max interpolation. IEEE Trans. Signal Process. 2003;51:560–574.
5. Pipe JG, Menon P. Sampling density compensation in MRI: Rationale and an iterative numerical solution. Magn. Reson. Med. 1999;41:179–186.
Figures