2950
Chemical Shift Encoded (CSE) Image Reconstruction for Spectral Selection in Fluorine-19 MRI1Medical Physics, University of Wisconsin - Madison, Madison, WI, United States, 2Radiology, University of Wisconsin - Madison, Madison, WI, United States, 3Electrical and Computer Engineering, University of Wisconsin - Madison, Madison, WI, United States, 4Radiology, Lausanne University Hospital (CHUV) and University of Lausanne (UNIL), Lausanne, Switzerland, 5Biomedical Engineering, University of Wisconsin - Madison, Madison, WI, United States
Synopsis
In preclinical applications, the high specificity of quantitative 19F MRI may be compromised by non-negligible signal contributions from fluorinated anesthetics (e.g. isoflurane). Here, we demonstrate the feasibility of chemical shift encoding (CSE) with multi-resonance fluorine signal modeling and least-squares estimation image reconstruction for 19F MRI. We optimize noise performance (NSA) and use a 3D spoiled gradient-echo acquisition to separate signal contributions from perfluoro-15-crown-5-ether (PFCE) and isoflurane. The method is tested in mixed PFCE/isoflurane phantoms showing effective signal separation. The CSE reconstruction removes isoflurane signal contributions in 19F MR images of PFCE in vivo, potentially reducing errors in 19F concentration quantification.
Introduction
Methods
Proposed CSE technique: A multi-TE CSE acquisition is proposed. The model used for CSE reconstruction (Equation 1) was solved using a non-linear least-squares estimation to determine the signal contributions ($$$ s $$$) at each k-space position ($$$ k_x,k_y,k_z $$$) from isoflurane ($$$ ρ_I $$$) and PFCE ($$$ ρ_P $$$).
$$ s_{TE} (k_x,k_y (t),k_z,t;ρ_P,ρ_I,f_B )=∭_{x,y,z}e^{i2πf_B (x,y,z)(TE+t)} e^{i2πxk_x} e^{i2πyk_y (t)} e^{i2πzk_z} [ρ_I (x,y,z) ∑_{m=1}^M(α_m e^{i2πf_m (TE+t)} ) +ρ_P (x,y,z) e^{i2πf_P (TE+t)} ]dx dy dz $$
The resonant frequencies of the PFCE ($$$ f_P $$$) and $$$ m $$$ isoflurane peaks ($$$ f_m $$$) with relative signal amplitude $$$ α_m $$$, the frequency shifts ($$$ f_B $$$) due to local B0 field inhomogeneity and phase evolution during the imaging readout ($$$ k_y(t) $$$, where $$$ t $$$ is the time relative to the $$$ TE $$$ during the readout) are included in the model.
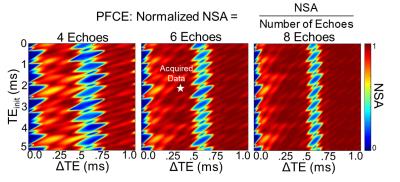
Noise performance optimization: Cramér-Rao Lower Bound (CRLB) analysis3 was performed in Matlab 2014b (MathWorks, Natick, MA) to determine the effective number of signal averages (NSA) at different combinations of initial echo time (TEinit), echo spacing (ΔTE), and number of echoes. NSA was normalized to the number of echoes.
Phantom experiment: All MR data were acquired on a 4.7T preclinical MRI system (Agilent Technologies, Santa Clara, CA). The spectral separation between PFCE and isoflurane was measured in phantoms of PFCE (Exfluoro, Round Rock, Tx) and isoflurane (Piramal, Bethlehem, PA) using a FID collected with a 90° global excitation, rBW=10kHz, and NSA=1. We demonstrated the feasibility of the technique in vitro with mixed phantoms of PFCE and isoflurane prepared in 0.5mL tubes, and MR imaged with a 3D SPGR repeated with multiple echo times (TR=10.0ms, TEinit=2.3ms, ΔTE=0.3ms, 12 TEs, 1.0mm3 isotopic resolution, rBW=50kHz, NSA=1) using a home-built 19F quadrature volumetric RF coil. CSE image reconstruction was performed in Matlab.
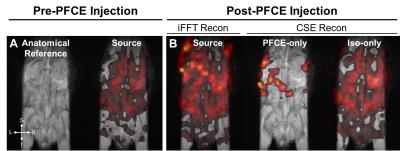
In vivo experiment: To demonstrate in vivo feasibility of CSE image reconstruction, MR data was collected on one healthy male C57BL/6 mouse anesthetized with 1.5% isoflurane and maintained at 37°C with a temperature probe and hot-air blower. This pilot study complied with institutional animal care and use committee regulations. The 19F MRI data was acquired with a 3D SPGR using the optimized TE and ΔTE from the CRLB analysis and phantom experiments. (TR=200.0ms, TEinit=2.3ms, ΔTE=0.3ms, 6 TEs, 1.6x1.6x4.0mm3 resolution, rBW=18kHz, NSA=8) prior to and after intraperitoneal injection of 45mM of a PFCE emulsion. The PFCE emulsion was synthesized as a kinetically stable, oil-in-water nanoemulsion loaded with PFCE. Anatomic 1H data was acquired with a T1-weighted 3D SPGR (TR=4.35ms, TE=2.19ms, and 0.31mm2 in-plane resolution.
Results
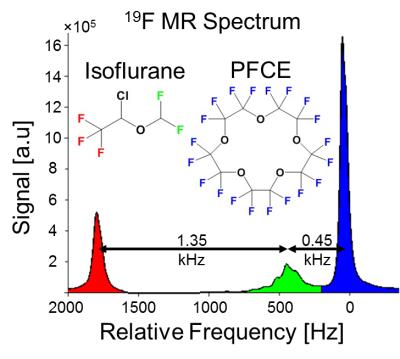
The relative chemical shifts between PFCE and isoflurane's CF3 and CHF2 spectral groups were 1.8kHz (9.6ppm) and 0.45kHz (2.4ppm) at 4.7T, respectively (Figure 1). CRLB analysis plots indicated optimal data acquisition parameters (TEinit, and ΔTE, and number of echoes) needed to maximize NSA for CSE reconstruction of PFCE and isoflurane (Figure 2). CSE reconstructed 19F images showed spectral separation of PFCE and isoflurane in mixed phantoms (Figure 3). The in vivo experiments demonstrated that fluorinated gas contamination can be removed from 19F MR images (Figure 4).Discussion
Conclusion
We have demonstrated the feasibility of a CSE approach for separation of PFCE and isoflurane in 19F MRI.Acknowledgements
The authors thank our collaborators and colleagues. We thank Alexa Barres and Dr. Sandro Mecozzi for providing the PFCE emulsion for in vivo studies. We gratefully acknowledge NIH awards UL1TR000427 and TL1TR000429, UW School of Medicine and Public Health, UW Carbone Comprehensive Cancer Center, and GE Healthcare for funding support.References
1Gaudet JM, Ribot EJ, Chen Y, Gilbert KM, Foster PJ. Tracking the fate of stem cell implants with fluorine-19 MRI. PLoS One. 2015;10(3):e0118544.
2Reeder SB, McKenzie CA, Pineda AR, Yu H, Shimakawa A, Brau AC, et al. Water-fat separation with IDEAL gradient-echo imaging. J Magn Reson Imaging. 2007;25(3):644-52.
3Pineda AR, Reeder SB, Wen Z, Pelc NJ. Cramer-Rao bounds for three-point decomposition of water and fat. Magn Reson Med. 2005;54(3):625-35.
4Brodsky EK, Holmes JH, Yu H, Reeder SB. Generalized k-space decomposition with chemical shift correction for non-Cartesian water-fat imaging. Magn Reson Med. 2008;59(5):1151-64.
5Temme S, Jacoby C, Owenier C, Grapentin C, Wang X, Schubert R, et al. Assessment of Thrombus Stage by 'Multicolor' 19F MRI. Proc Int Soc Magn Reson Med. 2016; 24.
6Basse-Lüsebrink TC, G. Ladewig, Kampf T, Melkus G, Haddad D, Bauer WR, et al. Multi-color 19F CSI: Simultaneous detection of differently labeled cells in vivo. Proc Int Soc Magn Reson Med. 2009;17.
7Jacoby C, Temme S, Mayenfels F, Benoit N, Krafft MP, Schubert R, et al. Probing different perfluorocarbons for in vivo inflammation imaging by 19F MRI: image reconstruction, biological half-lives and sensitivity. NMR Biomed. 2014;27(3):261-71.
Figures