2946
Protocol for investigating in vivo Glucose Metabolism in Human Breast Cancer by 13C MRS at 7T1Advanced Imaging Research Center, UT Southwestern Medical Center, Dallas, TX, United States, 2Radiology, UT Southwestern Medical Center, Dallas, TX, United States, 3Philips Medical Systems, Cleveland, OH, United States, 4Biomedical Engineering, Texas A&M University, TX, United States, 5Electrical Engineering, Texas A&M University, TX, United States, 6Chemistry, University of Texas at Dallas, TX, United States, 7Internal Medicine, UT Southwestern Medical Center, Dallas, TX, United States
Synopsis
Upregulated glucose uptake in cancer is often observed and can be monitored with a radiolabeled analogue of glucose, 18FDG, with detection by PET, however, a well-known constraint is its ionizing radiation. Additionally, except for the trapping of that glucose analogue, PET does not provide information about subsequent glucose metabolism. Here, we demonstrated the feasibility of a simple glucose infusion protocol that allows detection of glucose oxidation in human breast cancer in vivo via 7T 13C MRS. The [U-13C]glucose infusion is performed outside of the magnet making the protocol significantly more suitable for patients compared to previous approaches that required prolonged 13C substrate infusions inside the scanner.
Purpose
Aberrations in metabolic pathways in breast cancer have been studied in cell and rodent models but translating these results to humans is challenging. Developing technology for in vivo tumor metabolic characterization is thus desirable. Upregulated glucose uptake in cancer is often observed and can be monitored with a radiolabeled analogue of glucose, 18FDG, with detection by conventional PET or PET mammography (positron emission mammography, PEM). However, a well-known constraint of PET is its ionizing radiation which makes studies in younger subjects or serial exams for surveillance impractical. Even more critical is the shortcoming that, except for the trapping of that glucose analogue, PET does not provide information about subsequent glucose metabolism. Additionally, the specificity is limited, as positive PET findings do not always imply malignant lesions. Here, we examined the feasibility of direct detection of metabolism of [U-13C]glucose in the human breast using 13C MRS at 7T. The [U-13C]glucose infusion is performed outside of the magnet making the protocol significantly more suitable for patients compared to previous approaches that required prolonged 13C substrate infusions inside the scanner.Methods
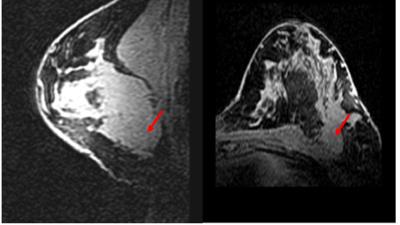
All procedures were performed with local IRB approval and after obtaining a written consent. In adult female volunteers (controls=2; breast cancer patient=1, Figure 1), [U-13C]glucose was administered through a peripheral intravenous line for 2 hours (outside of the magnet) in a protocol1,2 known to achieve a steady-state level of plasma glucose enrichment (50%) by administering a slow bolus of 8 grams followed by steady infusion at 8 grams/hour. After discontinuing infusion of glucose, proton-coupled 13C NMR spectra were acquired on a whole-body 7T scanner (Achieva, Phillips Medical Systems) with a 13C surface coil (8cm i.d. ) placed in a 1H T/R breast coil3. Non-localized 13C spectra were acquired in blocks averaging 64 acquisitions with TR = 4 s, (4 min, 16 sec), BW 16 kHz and 8k points. The spectra shown in Fig. 2 were acquired for approximately 40 min after discontinuing infusion of [U-13C]glucose. Baseline spectra with identical acquisition parameters were obtained for 4 min prior to glucose administration.Results and Discussion
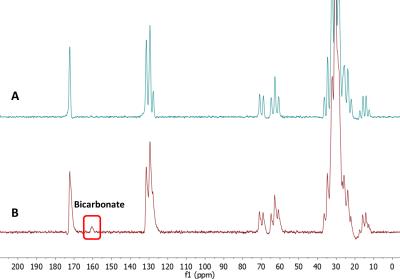
The infusion protocol was well tolerated by all patients. The subjects were administered [U-13C]glucose outside of the magnet while comfortably watching television or reading. The NMR observations were thus performed only after a high level of [U-13C] glucose was established in blood. Representative spectra are shown in Figure 2. A 13C-bicarbonate signal at ~161 ppm was detected post-infusion in the cancer patient but not in healthy controls. A natural abundance 13C-bicarbonate was not observed in the pre-infusion acquisitions in any of the subjects. This indicates that the 13C bicarbonate signal, post infusion, reflects active glycolysis of the labelled glucose to produce [U-13C]pyruvate followed by complete oxidation of the pyruvate in the TCA cycle to produce 13C-enriched HCO3-. The lack of this signal in healthy controls presumably reflects the low level of metabolic activity in normal, non-lactating, breast tissue. Routine clinical proton imaging is easily integrated into the current protocol (Figure 1).Conclusion
We demonstrated the feasibility of a simple glucose infusion protocol that allows detection of glucose oxidation in human breast cancer in vivo via 7T 13C MRS. We hypothesize that detection of a 13C bicarbonate signal at 161 ppm reflects complete oxidation of glucose via glycolysis and the TCA cycle in breast tumor. The sensitivity of this method could be improved in the future by implementing closely-fitting multinuclear receive breast arrays.Acknowledgements
This work was supported in part by grants from the National Institutes of Health (EB-015908 and HL-034557) and Cancer Prevention and Research Institute of Texas (RP150456).References
1. Maher EA, Marin-Valencia I, Bachoo RM, Mashimo T, Raisanen J, Hatanpaa KJ, Jindal A, Jeffrey FM, Choi C, Madden C, Mathews D, Pascual JM, Mickey BE, Malloy CR, DeBerardinis RJ. Metabolism of [U-13 C]glucose in human brain tumors in vivo. Nmr Biomed 2012;25(11):1234-1244.
2. Vikram Jakkamsetti , Levi Good, Dorothy Kelly, Sergey Cheshkov, Karthik Rajasekaran, Dean Sherry, Juan Pascual, Craig Malloy, and Ivan Dimitrov, “Exploring Human Brain Oxidative Metabolism and Neurotransmitter Cycling via coupled 13C MRS at 7T”, Proc. Intl. Soc. Mag. Res. Med. 23, 893 (2015).
3. Cui J, Bosshard J, Rispoli J, Dimitrov I, Cheshkov S, McDougall M, Malloy C, Wright S. A switched-mode breast coil for 7 tesla MRI using forced-current excitation. IEEE Trans Biomed Eng. 2015 Jul;62(7):1777-83.
Figures