2945
Localized, indirect 1H-[13C] MRS measurement of glutamate and glutamine 13C-labeling in frontal cortex of human brain at 4 Tesla.1Department of Radiology and Biomedical Imaging, Yale University, New Haven, CT, United States, 2Department of Endocrinology, Yale University, New Haven, CT, United States
Synopsis
We present an indirect 1H-[13C] MRS detection method (selective proton-observed, carbon edited (selPOCE) that can be used in the frontal lobe of human brain, allows for separation of overlapping glutamate (Glu) and glutamine (Gln) peaks and provides well-defined spatial localization of the 13C-signal. The method was successfully implemented at 4 Tesla and allowed for the selective detection of 13C-labeling in Glu and Gln in a timely manner (15 min), indicating that the selPOCE can be used for quantitative studies of brain energy metabolism and neurotransmission in a relatively small volume localized in the human frontal lobe.
Purpose
13C MRS in the frontal lobe of human brain during infusion of 13C-labeled substrates requires acquisition methods characterized by a low specific absorption rate (SAR). Avoiding high radiofrequency (RF) power deposition is critical for frontal lobe MRS applications because of the close proximity of the eyes. So far 13C MRS studies of frontal brain have relied on pulse-acquire-based sequences and 13C-labeling strategies that enrich the carboxylic carbons of brain metabolites (1,2). Because signals of natural abundance lipids from the scalp do not overlap with the peaks of interest detection of carboxylic carbons can be achieved without localization, thereby reducing the number of required RF pulses. Here we present an alternative indirect 1H-[13C] MRS detection method that can be used in the frontal lobe, allows for separation of overlapping glutamate (Glu) and glutamine (Gln) peaks, yet also provides well-defined spatial localization of the 13C-signal.
Methods
MRS. All MRS experiments were carried out on a 4 Tesla whole-body magnet interfaced to a Bruker Avance III HD spectrometer, running on Paravision 6 (Bruker Instruments, Billerica, MA, USA). The RF coil setup consisted of two circular surface coils positioned 10 mm apart in parallel planes: an outer 13C transmit only coil (∅ 135 mm) and an inner 1H transmit/receive coil (∅ 95 mm). MRS data acquisition relied on a selective proton-observed, carbon edited (selPOCE) pulse sequence. The selPOCE method is a combination between POCE based on stimulated echoes (3) and 2D HSQC (4). The conventional two-step POCE experiment is extended with two additional experiments that modulate the phase of two target signals (e.g. H4-Glu and H4-Gln), by utilizing the chemical shift difference on the 13C channel (e.g. 107 Hz between C4-Glu and C4-Gln at 4 Tesla). A similar approach was previously described for a HMQC-based variant on rat brain (5).
[1-13C]-Glucose infusion. A primed, variable infusion of [1-13C]-glucose (1.1M, 99% enriched, Cambridge Isotopes, Cambridge, MA, USA) was performed to rapidly reach plasma glucose concentrations of 10 mM. During the duration of the infusion (90 to 120 min), the plasma glucose level was allowed to gradually drop to ~7 mM.
Results
SAR. RF power output was measured at the RF amplifier and corrected for hardware losses. Average effective RF power output was 0.51 W for the 1H channel and 0.31 W for the 13C channel. Using the B1+ profiles of the RF coils for an estimation of the head volume affected by the B1+ field, we estimate to be significantly below the FDA guideline for average SAR (3W/kg).
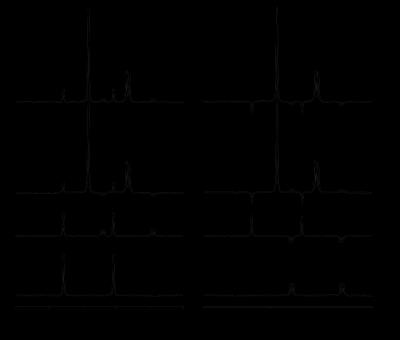
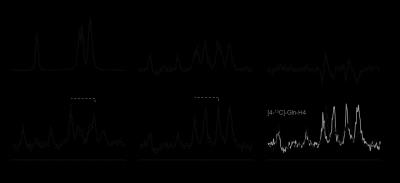
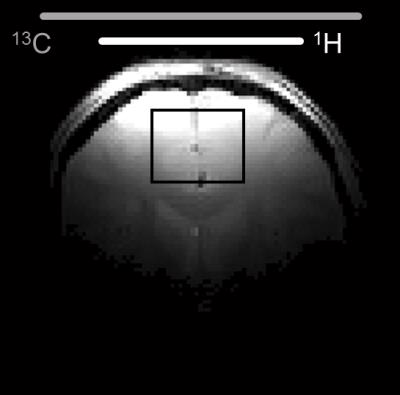
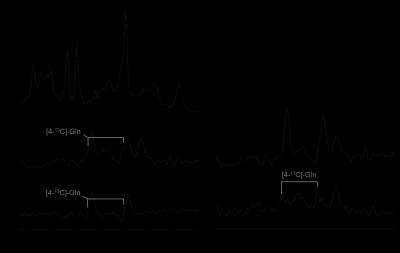
MRS. The selectivity of the pulse sequence for specific resonances is illustrated by data (Fig. 1) acquired in a phantom containing a mix of unlabeled and 13C-labeled acetate and lactate. The conventional POCE experiment (Fig. 1E and F) separates 13C-labeled from unlabeled resonances. The additional two scans of the selPOCE experiment modulate the phase of 1H signals, thereby allowing [3-13C]-lactate and [2-13C]-acetate to be separated based on their 13C chemical shift difference (Fig. 1G and H). Fig. 2 shows how this principle can be applied for the detection of [4-13C]-Glu-H4 and [4-13C]-Gln-H4, for which the difference in 1H chemical shift is only 0.11ppm (18.8 Hz at 4 Tesla). The 107 Hz difference on the 13C axis, allows robust separation of Glu and Gln H4 resonances. The faster in vivo T2* relaxation make the Glu and Gln overlap even more problematic as shown in the 1H MR spectrum acquired in the frontal lobe of a healthy volunteer (Fig. 4A). SelPOCE spectra acquired from a voxel (45 mL, Fig. 3) located in the frontal lobe during infusion of [1-13C]-glucose are shown in Fig. 4. Similar to the results obtained in the Glu-Gln phantom, combining individual in vivo selPOCE MR spectra generates difference spectra of all 13C-bound protons (Fig. 4B), as well as individual MR spectra of [4-13C]-Glu-H4 (Fig 4D) and [4-13C]-Gln-H4 (Fig 4E), respectively.
Discussion & Conclusion
The presented data illustrate the feasibility of a localized, 1H MRS-based 13C detection method that can be used to detect 13C-labeling of brain metabolites in the frontal lobe of human brain. The method was successfully implemented at 4 Tesla and allowed for the selective detection of 13C-labeling in Glu and Gln in a timely manner (15 min). In conclusion, selPOCE can be used for quantitative studies of brain energy metabolism and neurotransmission in a relatively small volume localized in the human frontal lobe.
Acknowledgements
We thank Terry Nixon, Scott McIntyre, and Peter Brown for maintenance and upgrades to the MR system.References
1. Li S, Zhang Y, Wang S, Yang J, Ferraris Araneta M, Farris A, Johnson C, Fox S, Innis R, Shen J. In vivo 13C magnetic resonance spectroscopy of human brain on a clinical 3 T scanner using [2-13C]glucose infusion and low-power stochastic decoupling. Magn. Reson. Med. 2009;62:565–573. doi: 10.1002/mrm.22044.
2. Li S, Zhang Y, Wang S, Araneta MF, Johnson CS, Xiang Y, Innis RB, Shen J. 13C MRS of occipital and frontal lobes at 3 T using a volume coil for stochastic proton decoupling. NMR Biomed. 2010;23:977–985. doi: 10.1002/nbm.1524.
3. Pfeuffer J, Tkác I, Choi I-Y, Merkle H, Ugurbil K, Garwood M, Gruetter R. Localized in vivo 1H NMR detection of neurotransmitter labeling in rat brain during infusion of [1-13C] D-glucose. Magn. Reson. Med. 1999;41:1077–1083. doi: 10.1002/(SICI)1522-2594(199906)41:6<1077::AID-MRM1>3.0.CO;2-#.
4. de Graaf RA, De Feyter HM, Rothman DL. High-sensitivity, broadband-decoupled 13C MR spectroscopy in humans at 7T using two-dimensional heteronuclear single-quantum coherence. Magn. Reson. Med. 2014:n/a-n/a. doi: 10.1002/mrm.25470.
5. Henry P-G, Roussel R, Vaufrey F, Dautry C, Bloch G. Semiselective POCE NMR spectroscopy. Magn. Reson. Med. 2000;44:395–400. doi: 10.1002/1522-2594(200009)44:3<395::AID-MRM9>3.0.CO;2-5.
Figures