2944
2D Heteronuclear Single-Quantum Coherence MR spectroscopy for in vivo detection of 13C-labeling in rat brain during simultaneous infusion of 13C-labeled substrates1Department of Radiology and Biomedical Imaging, Yale University, New Haven, CT, United States, 2Department of Psychiatry, Yale University, New Haven, CT, United States
Synopsis
We adopted a 2D heteronuclear single-quantum coherence (HSQC) MR spectroscopy method to detect in vivo 13C isotopomers in rat brain through exploiting the high sensitivity of 1H MRS. This method allows for in vivo detection of unique 13C-labeling patterns in brain metabolite pools during simultaneous infusion of different 13C-labeled substrates. We demonstrated that high-quality 2D HSQC MR spectra can be acquired in vivo in a time-resolved manner from rat brain during simultaneous infusion of [U-13C6]-glucose and [2-13C]-acetate. This method can be used to study with high accuracy neuronal and glial metabolism, and the contribution of alternate substrates to brain energy metabolism.
Purpose
13C MR isotopomer analysis is a powerful method to reveal contributions of different metabolic pathways and substrates in biological systems (1,2). In vivo detection of 13C isotopomers is challenging due to the intrinsic low sensitivity of 13C NMR, a consequence of its relatively low gyromagnetic ratio. We set out to adopt a 2D heteronuclear single-quantum coherence (HSQC) MR spectroscopy method to detect in vivo 13C isotopomers in rat brain originating from different 13C-labeled substrates, while exploiting the high sensitivity of 1H MRS. We show that successful implementation of this method allows for detection of unique 13C-labeling patterns in brain metabolite pools during simultaneous infusion of 13C-labeled glucose and acetate.
Methods
MRS. All MRS experiments were performed on an 11.7 Tesla magnet interfaced to a Bruker Avance 3 HD spectrometer, running Paravision 6 (Bruker Instruments, Billerica, MA, USA). A combined quadrature 13C and single loop 1H surface radiofrequency coil setup was used to acquire MR spectra from a 192 μL voxel containing predominantly cortical tissue. Animals were anesthetized using isoflurane and ventilated following tracheotomy. Venous and arterial catheters were placed for the 120 min infusion of [U-13C6]-glucose (1.1M) and [2-13C]-acetate (2M), and blood sampling and blood pressure monitoring, respectively. All animal experiments were approved by the Yale University IACUC.
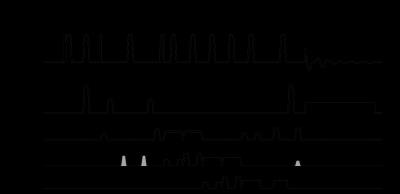
HSQC sequence. Figure 1 shows the HSQC sequence based on 3D LASER (3) localization. The orthogonal phase relation between the first and second proton excitation pulses is critical for optimal signal recovery. We therefore avoided gradient pulses during the preparation phase and based 1H excitation and refocusing on hard RF pulses and a pair of adiabatic full passage (AFP) pulses, respectively, for their predictable phase behavior. Coherence selection was based on magnetic field gradients with 2:2:1 areas (gray gradient along y in Fig. 1). To cover the entire 13C spectral width, 13C excitation pulses were executed as 1.0 ms AFP pulses (HS4 modulation (4), 25 kHz bandwidth) operated in the non-adiabatic power regime. Broadband decoupling was based on 1.6 ms AFP pulses (HS4 modulation, 12.5 kHz bandwidth) incorporated in a 20-step decoupling supercycle (5). A 2D HSQC spectrum was created by incrementing the t1 evolution time through 192 steps of 0.5 ms. To reduce the experimental duration, the repetition time TR was decreased from 4,000 to 1,000 ms with increasing t1 evolution delays (6), giving a time resolution of 12 min per spectrum.
Results
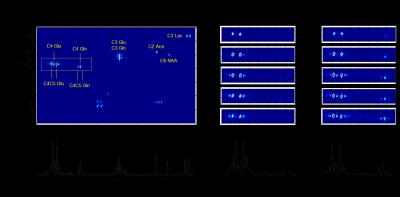
Figure 2A shows a 2D HSQC spectrum acquired from rat brain (192 mL) between 60 and 100 min following the infusion of [U-13C6]-glucose and [2-13C]-acetate. A number of well-separated signals can be recognized at the intersection of their corresponding 1H and 13C chemical shifts. For example, the cluster around 34.2 and 2.34 ppm are resonances associated with C4-labeled glutamate (Glu) which include [4-13C]-Glu, [4,5-13C2]-Glu and [3,4,5-13C3]-Glu. Note that the projection onto the 1H chemical shift axis, as would be obtained with classical proton-observed, carbon-edited (POCE) MRS, does not allow a differentiation of the various isotopomers. A projection on the 13C axis provides a classical, proton-decoupled 13C MR spectrum, albeit at the higher proton sensitivity. Fig. 2B and 2C show time-resolved HSQC spectra following the intravenous infusion of (B) [U-13C6]-Glc only and (C) combined [U-13C6]-Glc and [2-13C]-acetate. It follows that the doublet [4,5-13C2]-Glu and Gln resonances are uniquely associated with the [U-13C6]-Glc substrate, whereas the singlet [4-13C]-Glu and Gln are directly linked with [2-13C]-acetate metabolism.
Discussion
The data gathered with the described 2D HSQC method indicate that 13C MRS spectra can be acquired in vivo while making use of the high sensitivity of 1H MRS. The spectral resolution is sufficient to discriminate singlet and multiplet resonances of a specific metabolite. This allows for simultaneous infusion of substrates that are 13C-labeled in such a way that they uniquely lead to 13C MR singlets or multiplets in brain metabolite pools within the time span of the infusion. The spectra can be acquired at a time resolution appropriate for kinetic studies, as indicated by the time course data. Given the relative sparsity of the number of resonances in the 2D HSQC spectrum there is still room to reduce the number of t1 evolution delays thereby shortening the total acquisition time.
Conclusion
It was demonstrated that high-quality 2D HSQC MR spectra can be acquired in vivo from rat brain during simultaneous infusion of two 13C-labeled substrates. This method can be used to study with high accuracy neuronal and glial metabolism, and the contribution of several alternate substrates to brain energy metabolism.
Acknowledgements
We thank Bei Wang for assistance with animal preparation and Terry Nixon, Scott McIntyre, and Peter Brown for maintenance and upgrades to the MR system.References
1. Malloy CR, Sherry AD, Jeffrey FM. Analysis of tricarboxylic acid cycle of the heart using 13C isotope isomers. Am. J. Physiol. - Heart Circ. Physiol. 1990;259:H987–H995.
2. Henry P-G, Öz G, Provencher S, Gruetter R. Toward dynamic isotopomer analysis in the rat brain in vivo: automatic quantitation of 13C NMR spectra using LCModel. NMR Biomed. 2003;16:400–412. doi: 10.1002/nbm.840.
3. Garwood M, DelaBarre L. The Return of the Frequency Sweep: Designing Adiabatic Pulses for Contemporary NMR. J. Magn. Reson. 2001;153:155–177. doi: 10.1006/jmre.2001.2340.
4. Tannus A., Garwood M. Improved performance of frequency-swept pulses using offset-independent adiabaticity. J Magn Reson A 1996:133–137.
5. de Graaf RA. Theoretical and experimental evaluation of broadband decoupling techniques for in vivo nuclear magnetic resonance spectroscopy. Magn. Reson. Med. 2005;53:1297–1306. doi: 10.1002/mrm.20507.
6. de Graaf RA, De Feyter HM, Rothman DL. High-sensitivity, broadband-decoupled 13C MR spectroscopy in humans at 7T using two-dimensional heteronuclear single-quantum coherence. Magn. Reson. Med. 2014:n/a-n/a. doi: 10.1002/mrm.25470.
Figures