2943
Analyzing Reaction Dynamics With Hyperpolarized 13C-NMR1Radiology, University of Pennsylvania, Philadelphia, PA, United States
Synopsis
In this study we use hyperpolarized 13C-NMR to probe the dynamics of the decarboxylation reaction of pyruvate via H2O2, commonly used to produce hyperpolarized bicarbonate. Using this method we are able to observe and quantify the dynamics of the intermediate state, 2-hydroperoxy-2-hydroxypropranoate, which has never before been directly observed at room temperature, as well as characterizing a previously overlooked side reaction between the products and reactants of the decarboxylation reaction. This study serves as a template for how to use hyperpolarized 13C NMR to study the dynamics of innumerable other organic reactions with polarizable substrates.
Introduction
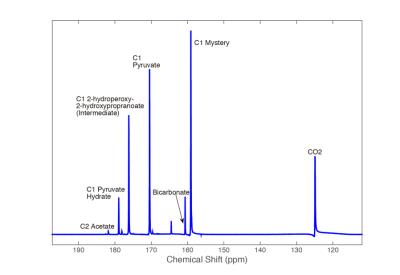
The study of transient intermediate states in chemical reactions has long proven difficult for chemists. NMR’s usefulness is limited in in this arena due to the low thermal polarization of atomic nuclei, which yields poor temporal resolution. In this study, we demonstrate that the NMR signal enhancement provided by hyperpolarized (HP) 13C NMR can be used to probe the dynamics of these intermediate states and lead to the quantification of reaction kinetics. HP 13C-bicarbonate, often used for pH imaging experiments, can be produced through the decarboxylation of HP [1-13C] pyruvate. However some aspects of this reaction are poorly understood; the proposed intermediate state, 2-hydroxyperoxy-2-hyroxypropranoate, has never been directly observed at room temperature, and a mysterious peak is often seen in spectra ~1.5ppm to the right of bicarbonate which has been previously assumed to be an impurity or secondary intermediate state [1]. Here we report the first observation of this reaction’s intermediate state at room temperature, as well as the characterization of the ‘mystery’ peak as peroxycarbonate, the product of a side reaction between CO2 and the hydroperoxide ion, OOH-.Methods
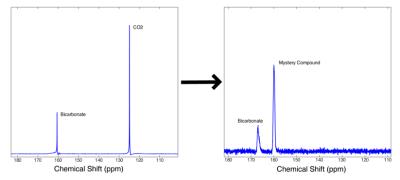
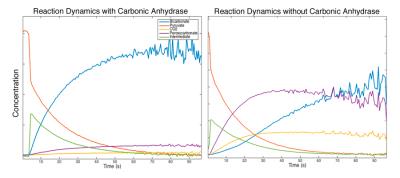
All reactions were preformed by sequentially injecting reactants into a 20mm NMR tube placed in a 9.4T vertical bore magnet (Varian Inc). In the initial reaction analysis experiments, 28.6mg of [1-13C]-pyruvate was polarized to ~25% in a Hypersense DNP polarizer (Oxford Instruments) and quickly injected into the 20mm reaction tube where it was diluted to 6.7mM in 15mL of DI water with 100mM of tris, CHES, or CAPS buffer, depending on the target pH of the particular experiment. 80mM of H2O2 was injected into the reaction mixture <5 seconds after the HP pyruvate solution to initiate the reaction. Spectra were acquired for at least 100 seconds with a TR of 250ms and a flip angle of 10°. To help determine the identity of the compound producing the ‘mystery’ peak, this same experiment was repeated using [2-13C]-pyruvate. In order to confirm that this compound was indeed the product of a side reaction between CO2 and the hydroperoxide ion, two additional reaction experiments were preformed. In the first experiment, directly polarized 13C-bicarbonate was mixed with H2O2 at elevated pH in the absence of pyruvate, the appearance of a peak at the same chemical shit as the ‘mystery’ peak would confirm that it is the result of a reaction between bicarbonate or CO2 and H2O2 or OOH-. In the second experiment, the decarboxylation reaction was repeated at elevated pH in the presence of >600 units/mL of carbonic anhydrase to assess the change in the ‘mystery’ compound dynamics when the produced CO2 is rapidly converted to bicarbonate. All data was analyzed in custom Matlab software. A model of the full reaction dynamics was developed and implemented in Matlab.Results
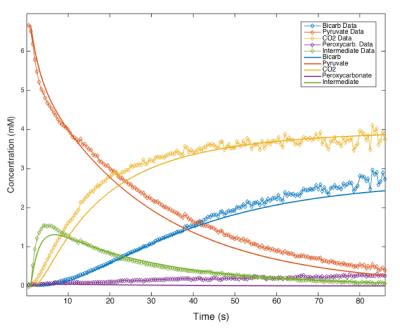
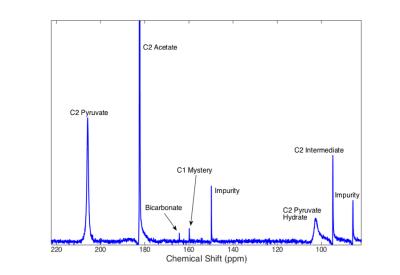
Using hyperpolarized 13C-NMR, we are able to acquire spectra of reactions in progress with temporal resolution as low as 100ms without significant loss in spectral resolution. As seen in Figure 1, 2, & 5, we have more than enough signal to directly quantify the dynamics of the intermediate state and we find it at the same chemical shift as previously reported in low temperature studies [2]. This constitutes the first direct observation of this intermediate state at room temperature. From the spectra of the decarboxylation reaction of [2-13C] pyruvate shown in Figure 3, we see all of the expected 2C peaks except for one that could represent the mystery compound. From this we conclude that the compound must contain just a single carbon atom. We theorized that this compound could be the peroxycarbonate ion, formed from the reaction of CO2 and OOH-. As shown in Figure 4, when H2O2 and NaOH are mixed with a solution of hyperpolarized 13C-bicarbonate and 13CO2, peroxycarbonate is produced at the same chemical shift as the ‘mystery’ compound. When carbonic anhydrase was introduced into the decarboxylation reaction at elevated pH, the production of peroxycarbonate is hindered due to the rapid conversion of CO2 to bicarbonate as shown in Figure 5. A model of the reaction was developed based on the results of these experiments, which provided reasonably good fits as seen in Figure 2.Conclusions
We have used hyperpolarized 13C-NMR to thoroughly investigate and characterize the dynamics of the decarboxylation reaction of pyruvate via H2O2, a reaction often used for the production of high polarization 13C-bicarbonate. This study could serve as a template for using hyperpolarized 13C NMR to study the dynamics of innumerable other organic reactions with polarizable substrates, and demonstrates the wealth of information potentially available to chemists from such studies.Acknowledgements
This work was supported by the National of Institutes of Health (NIH) R01 HL124986.References
[1] Ghosh, R. K., Kadlecek, S. J., Pourfathi, M. & Rizi, R. R. Efficient production of hyperpolarized bicarbonate by chemical reaction on a DNP precursor to measure pH. Magn. Reson. Med. 74, 1406–1413 (2015).
[2] Asmus, C., Mozziconacci, O. & Schöneich, C. Low-Temperature NMR Characterization of Reaction of Sodium Pyruvate with Hydrogen Peroxide. J. Phys. Chem. A 119, 966–977 (2015).
Figures