2940
Ferumoxytol as a blood-pool T2 relaxation agent for 7T phosphorus spectroscopy1Department of Physiology, Anatomy & Genetics, University of Oxford, Oxford, United Kingdom, 2Oxford Centre for Clinical Magnetic Resonance Research, University of Oxford, Oxford, United Kingdom, 3Department of Physics, University of Oxford, Oxford, United Kingdom
Synopsis
Ferumoxytol is a licensed carbohydrate-coated, superparamagnetic iron oxide nanoparticle indicated in the treatment of anaemia. We show that, in contrast to other agents, it predominantly reduces T2, is confined to the blood pool for >1 hour post administration, and therefore could improve the efficiency of saturation pulses that aim to remove the 2,3-diphosphoglycerate signal from blood. This proof-of-principle study shows that Ferumoxytol could enable inorganic phosphate detection in vivo, and hence the determination of pH.
Purpose
31P MRS is an invaluable tool to probe myocardial metabolism.[1] The accurate determination of the chemical shift of the inorganic phosphate (Pi) peak in 31P MRS enables the quantification of intracellular pH,[2] but the determination of myocardial pH in vivo is challenging due to the large and spectrally overlapping 2,3-diphosphoglycerate (2,3-DPG) resonance in the blood pool, even at 7T.[3] Whilst the use of CSI ameliorates this effect significantly, it cannot be completely removed due to partial volume and incomplete saturation effects and the presence of vasculature in the myocardium.[4] Ferumoxytol is a carbohydrate-coated, superparamagnetic iron oxide nanoparticle indicated in the treatment of anaemia that recently has been shown to act as an effective MR negative contrast agent[5] that is confined to the blood pool.[6] We wished to investigate the feasibility of using Ferumoxytol as a T2-reducing agent to suppress unwanted 2,3-DPG signal in the blood pool.Methods
All experiments were performed with local ethical approval on a 7T Varian DDR system.
In vitro experiments: Fresh healthy human blood ($$$3\times2.7\,\text{mL}$$$ vials) was acquired via antecubital fossa venipuncture, and stored in sodium citrate buffer (Vactutainer, BD Healthcare). A cannulation tube was inserted to allow the in situ introduction of Ferumoxytol at the clinically indicated dose (4 mg/kg, or [Fe]$$$=0.9\,\text{mM}$$$ in blood). 31P hard pulse spectra were acquired via a single loop surface coil (TR$$$=512\,\text{ms}$$$;$$$\,10\,\text{kHz}$$$ bandwidth; 3840 averages; $$$\theta_\text{Ernst}\approx30^\circ$$$ nominal flip angle) before and after addition of Ferumoxytol. Proton T2 was also measured by CPMG prior and post Ferumoxytol addition, and T1 by inversion recovery in both blood and serial dilutions of Feruomoxytol in saline.
In vivo demonstration: A healthy male Wistar rat (200g) was anaesthetised (isoflurane, 2% in $$$2\,\text{L/min}$$$ O2) and cannulated (tail vein). As an intermediate step to investiate the cardiac half-life of Ferumoxytol, short-axis proton CINE images of the heart were acquired ($$$51.2\times51.2\,\text{mm}^2$$$ FOV; $$$96\times96$$$ matrix; $$$1.2\,\text{mm}$$$ slice thickness; $$$20^\circ$$$ FA; 4 averages; TR$$$=4.16\,\text{ms}$$$; TE$$$=1.68\,\text{mm}^2$$$; $$$72\,\text{mm}$$$ volume coil) continuously prior to and up to one hour after the administration of Ferumoxytol. A short T1 phantom was included next to the animal as a signal reference.
Results
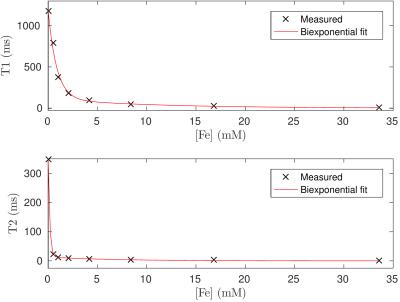
Ferumoxytol is an efficient relaxation agent: As illustrated in Fig. 1, Ferumoxytol reduced saline T1 and T2 from $$$1183/362\,\text{ms}$$$ to $$$790/28\,\text{ms}$$$ at the physiological concentration of $$$0.5\,\text{mM}$$$. In saline, this corresponds to a reduction in T1/T2 by a factor of 1.7/12.7. The observed behaviour was well described by a double exponential model, with short/long components of $$$1200/88\,\text{mol}^{-1}$$$ for T1 and $$$6600/310\,\text{mol}^{-1}$$$ for T2.
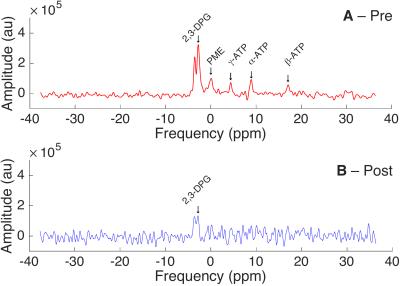
Reduction of blood pool signal: The T2 of fresh blood decreased by nearly an order of magnitude, from $$$65\,\text{ms}$$$ to $$$5.2\,\text{ms}$$$ following the addition of Ferumoxytol, whereas T1 reduced from approximately $$$800\,\text{ms}$$$ to $$$400\,\text{ms}$$$. As a direct consequence, Ernst-angle hard pulse spectroscopy on whole human blood showed a reduction in all metabolites. The baseline-corrected integrated DPG peak amplitude reduced from $$$7\times10^5$$$ to $$$1\times10^5$$$. (Fig. 2)
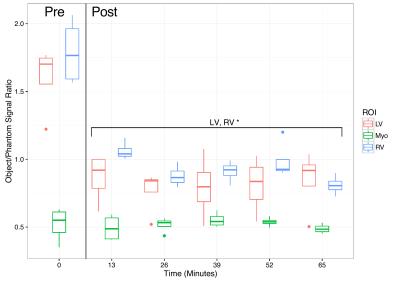
Ferumoxytol is a blood-pool agent that is MR visible for $$$>1$$$ hour: Repeated CINE measurements show a uniform decrease in LV and RV signal compared to that of the phantom that is statistically significant, but no change in the myocardial signal (Fig. 3, $$$p<10^{-7}$$$ by ANOVA). Given that the received signal under these conditions is proportional to T2 in the regime that $$$T_1\gg\text{TR}$$$ this observed effect is consistent with a predominantly T2 mediated effect, as has been reported elsewhere.[7]
Discussion and Further Work
This proof-of-principle study has demonstrated that Ferumoxytol substantially reduces T2 at 7T, and is confined to the blood pool in the in vivo rodent heart. The value observed at 7T of R2 compared to R1 is consistent with the frequency dependence of R2 and R1 reported for other USPIOs of $$$10\,\text{nm}$$$ diameter at lower fields.[8] As a direct consequence of reducing T2 the detected phosphorous signal observed by hard pulse spectroscopy decreased by a factor of seven, despite the fact that pulse/acquire experiments are not the most appropriate sequence to maximally exploit this change. Therefore, future work will demonstrate the utility of Ferumoxytol in small animals. CSI experiments are likely to be significantly improved by the addition of Ferumoxytol as the reduction in T2 would dramatically increase the efficiency of saturation pulses or preparation schemes such as MLEV pulses. As Ferumoxytol is licensed for use in humans, it could therefore form a translatable negative-contrast agent to enhance the ability of 31P-MR to resolve the inorganic phosphate peak in the myocardium without the large contaminant signal from 2,3-DPG in the blood pool, and hence determine myocardial pH. Future work will demonstrate this effect in vivo.Acknowledgements
We would like to acknowledge financial support from the British Heart Foundation (FS/10/002/28078; FS/14/17/30634; RG/11/9/28921), a Sir Henry Dale Fellowship from the Wellcome Trust and the Royal Society (098436/Z/12/Z), and an EPSRC Doctoral Prize Fellowship (EP/M508111/1).References
[1] D. J. Tyler, L. E. Hudsmith, K. Clarke, S. Neubauer, M. D. Robson, NMR Biomed. Oct. 2008, 21, 793– 798.
[2] R. B. Moon, J. H. Richards, J. Biol. Chem. 1973, 248, 7276–7278.
[3] C. T. Rodgers, W. T. Clarke, C. Snyder, J. T. Vaughan, S. Neubauer, M. D. Robson, Magn. Reson. Med. Aug. 2014, 72, 304–315.
[4] J. R. Gober, G. G. Schwartz, S. Schaefer, B. M. Massie, G. B. Matson, M. W. Weiner, G. S. Karczmar, Magn. Reson. Med. Aug. 1991, 20, 171–183.
[5] A. Pohlmann, P. Karczewski, M.-C. Ku, B. Dieringer, H. Waiczies, N. Wisbrun, S. Kox, I. Palatnik, H. M. Reimann, C. Eichhorn, S. Waiczies, P. Hempel, B. Lemke, T. Niendorf, M. Bimmler, NMR Biomed. Sept. 2014, 27, 1085–1093.
[6] T. Christen, W. Ni, D. Qiu, H. Schmiedeskamp, R. Bammer, M. Moseley, G. Zaharchuk, Magn. Reson. Med. Sept. 2013, 70, 705–710.
[7] S. R. Alam, C. Stirrat, J. Richards, S. Mirsadraee, S. I. K. Semple, G. Tse, P. Henriksen, D. E. Newby, J. Cardiovasc. Magn. Reson. 2015, 17, 83.
[8] P. Arosio, J. Thévenot, T. Orlando, F. Orsini, M. Corti, M. Mariani, L. Bordonali, C. Innocenti, C. San- gregorio, H. Oliveira, S. Lecommandoux, A. Lascialfari, O. Sandre, J. Mater. Chem. B 2013, 1, 5317.
Figures