2939
Exogenous NAD+ Enhances Energy Metabolism in Healthy Rat Brains1Center for Magnetic Resonance Research, University of Minnesota, Minneapolis, MN, United States
Synopsis
Decline in NAD+ availability is tightly linked to many neurological disorders. Our recent study also revealed age dependences of intracellular NAD+, NADH and total NAD concentrations in healthy human brains. Accumulating evidences have shown that the cellular NAD+ could serve as a therapeutic target for treating metabolic or age-related neurological diseases and promoting longevity. Therefore, to investigate the effect of exogenous NAD+ on intracellular NAD metabolism, the in vivo 31P-MRS NAD imaging assay developed in our lab was applied in normal rat brains at 16.4 T. Significant increases of cerebral α-ATP, total NAD and NAD+ levels were observed after the intra-peritoneal infusion of exogenous NAD+. This study not only demonstrates the feasibility of using exogenous NAD+ to enhance cerebral ATP and NAD metabolisms, but also provides an opportunity to better understand the roles of NAD metabolism in health and age-related disease.
Introduction
Nicotinamide adenine dinucleotide (NAD) exists in oxidized (NAD+) or reduced (NADH) form in all living cells and mediates ATP energy production through the NAD+/NADH redox state, which reflects the metabolic balance between mitochondrial oxidation and cytosolic glycolysis. NAD+ also modulates metabolic signaling via regulating the activities of NAD+-dependent enzymes; thus, participates in many important cellular processes including aging, neurodegeneration and cell death (1-3). It is known that decline in NAD+ availability is tightly linked to age-related neurological disorders. Our recent study also revealed age dependences of intracellular NAD+, NADH and total NAD concentrations in healthy human brains (4). Accumulating evidences have demonstrated that the cellular NAD+ could serve as a therapeutic target for treating metabolic or age-related neurological diseases and promoting longevity (5). Therefore, to investigate the effect of exogenous NAD+ on intracellular NAD metabolism, in this study, the in vivo 31P-MRS NAD imaging assay developed in our lab (6) was applied in normal rat brains at 16.4 T. Dynamic localized 31P spectra were acquired using 3D-chemical shift imaging (CSI) technique and changes in cerebral levels of a-ATP and NAD were examined due to the intra-peritoneal (i.p.) infusion of exogenous NAD+.Method
Seven male Sprague Dawley rats (body weight (BW): 371±31 g) were anesthetized by 2% isoflurane with their femoral arteries catheterized for blood sampling and physiological monitoring. All MR experiments were conducted at 16.4 T/26 cm scanner (Varian/VNMRJ) using a passively decoupled 1H/31P surface coil. Dynamic 3D-31P CSI data of rat brains were acquired with 87 μL nominal spatial and 9 min temporal resolutions for about 360 min to monitor the metabolites changes before, during and after the NAD+ infusion. For each rat, 30 min baseline data were acquired that followed by a 15-min i.p. infusion of NAD+ (400 mg/kg BW, Sigma-Aldrich). A 20 Hz linebroadening was used before Fourier transformation to enhance spectral signal-to-noise ratio. The concentrations of α-ATP and NAD metabolites including NAD+, NADH and total NAD ([NAD+]+[NADH]) were quantified using in vivo 31P-MRS NAD assay as previously described (6).Result
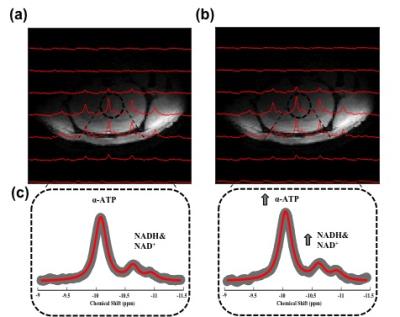
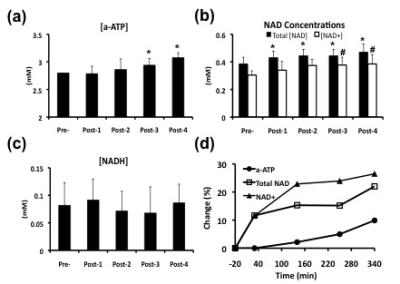
Figure 1a-b displays the anatomical image of a representative rat brain overlaid with 31P CSI spectra at 20 min before and 340 min after exogenous NAD+ infusion. The outstanding quality of the localized spectra allowed sensitive detection and excellent model fittings of the α-ATP and NAD resonances (Fig. 1c), resulting in reliable quantifications. As shown in Figure 1c, significant enhancements of the α-ATP and NAD signals were observed due to the NAD+ infusion. Figure 2 illustrates the effects of the NAD+ infusion on α-ATP, NAD+, NADH and total NAD concentrations in normal rat brains. Significant increases of the cerebral α-ATP and NAD+ levels were observed from 250 min after the exogenous NAD+ infusion (Fig. 2a-b). Total NAD concentration significantly increased after 30 min of the infusion (Fig. 2b) and there was no significant change of NADH content during the whole experimental procedure (Fig. 2c). Figure 2d summarizes the percentage increases of α-ATP, total NAD and NAD+ levels, demonstrating the maximum of 10%, 22% and 26% at 340 min after the infusion, respectively.Discussion and Conclusion
This study demonstrates for the first time the feasibility of exogenous NAD+ in regulating cerebral ATP and NAD metabolisms in healthy rat brains. The significant increases of α-ATP and total NAD concentrations indicate an enhancement of cerebral energy metabolism induced by exogenous NAD+. These findings evidence the potential of NAD+ to be a target for treating metabolic or age-related neurological disorders via neuroenergetic improvement. The acute effects observed in this work would lay a foundation for future studies focusing on the chronic effect of exogenous NAD+ and/or its precursors on promoting longevity in animal and human brains. In summary, our recently developed 31P-MRS NAD imaging technique is particularly useful for examining the in vivo NAD metabolism, which provides an opportunity to better understand the roles of NAD in health and age-related disease.Acknowledgements
NIH Grants: R01 NS41262, NS57560, NS70839, MH111447, R24 MH106049, P41 EB015894, P30 NS076408, S10 RR025031 and Keck foundation.References
(1) Mouchiroud, L. et al. (2013) Cell, 152(2): 430-441. (2) Pittelli, M. et al. (2011) Mol Pharmacol, 80(6): 1136-1146. (3) Wiley, C. et al. (2014) EMBO J, 33(12): 1289-1291. (4) Zhu, X. et al. (2015) Proc Natl Acad Sci USA, 112(9): 2876-2881. (5) Houtkooper, R. et al. (2012) J Cell Biol, 199(2): 205-209. (6) Lu, M. et al. (2014) Magn Reson Med., 71(6): 1959-1972.Figures