2935
Dynamic 31P spectroscopy during superimposed electrical muscle stimulation and volitional contraction for enhanced metabolic response in the skeletal muscle1Division of Radiological Physics, University of Basel Hospital, Basel, Switzerland, 2Department of Biomedical Engineering, University of Basel, Basel, Switzerland, 3Pediatric Neurology, UniversitätsKinderspital beider Basel (UKBB), Basel, Switzerland
Synopsis
Dynamic 31P spectroscopy of the skeletal muscle can provide useful insight into its energy metabolism. However, in order to see dynamic changes in metabolites, a minimum threshold of physical exercise is necessary. In this work, we present a system that uses electrical muscle stimulation superimposed to volitional muscle contraction in order to enhance the metabolic response of the muscle in the same workload condition. This method can have potential application to patients that are unable to voluntarily exert sufficient work for a dynamic spectroscopy investigation.
Introduction
31P spectroscopy during exercise is a valuable method to investigate muscle energy metabolism. However, in order to yield significant metabolic changes, a minimum level of induced muscle fatigue is necessary, which could be challenging for patients with premature fatigue or other neuromuscular impairments. While electrical muscle stimulation (EMS) can be a valid alternative to volitional exercise1, the type of elicited response is not exactly the same. It has been proposed2,3 that superimposing EMS to volitional exercise can enhance the metabolic response while maintaining other exercise parameters constant. In this work, we present a design for the acquisition of dynamic 31P spectra during superimposed EMS and volitional exercise (a protocol that we name SEMS for brevity) and show an in vivo feasibility test of this system at 3T.Methods
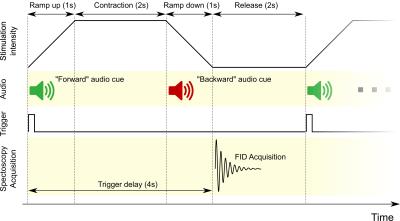
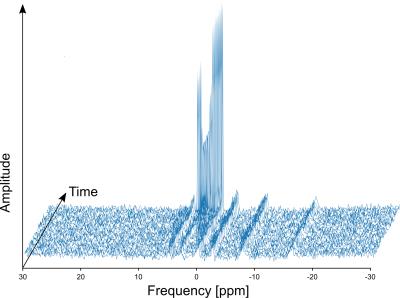
A custom system was developed for the acquisition of 31P spectra during SEMS, comprising a commercial, low-cost dual-channel electrical muscle stimulator connected to a custom microcontroller-based circuit. The circuit was responsible for generating a trigger signal synchronized with the stimulation and to communicate with an external computer via optical fiber serial connection. The external computer was used to enable/disable the delivery of the stimulus to the muscle and to generate audio cues synchronized with the stimulation. The acquisition was performed on a 3T whole-body scanner equipped with a dual-tuned 1H/31P surface coil and a commercial MR-safe air-pressure-operated ergometer for the activation of the triceps surae (plantar flexion). After preliminary experiments to establish the mutual interference between EMS and acquisition, two exercise sessions were performed on different days. Non-localized FIDs were acquired in rest for saturation correction (TR/TE: 15000/0.35ms, averages: 16, flip angle: 42°, vector size: 1024, bandwidth: 3000Hz). γATP was used for calculation of absolute concentration. Next, a standard exercise protocol (2min rest, 5min exercise, 5min recovery) was executed and dynamic spectra were acquired. EMS was delivered through saline-based electrodes placed on the triceps surae during the exercise phase, with a periodic stimulation with a 6s period (i.e. 10 extensions per minute). Audio cues were presented to the subject in synchronization with the stimulation (fig. 1). Dynamic spectra were acquired at each release phase with the following parameters: TR/TE: 6000/0.35ms, averages: 1, flip angle: 70°, vector size: 512, bandwidth: 3000Hz, measurements: 120. In each scanning session, the acquisition was repeated twice with a rest of 20 minutes in-between, first with SEMS, and secondly with volitional contraction only. On the first day, the EMS intensity was set to 20mA; on the second day, it was set to 25mA. The pressure of the ergometer was set to 0.90 bar. Quantification of the spectra was performed with the AMARES algorithm and then the data were postprocessed to extract: pH, phosphocreatine (PCr) and inorganic phosphate (Pi) time courses, PCr depletion, maximal oxidative flux (Qmax). The ADP concentration was calculated according to Kemp et al.4,5 assuming that 15% of the total Creatine was not phosphorylated.Results
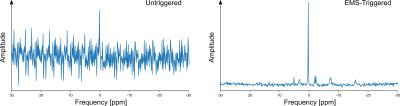
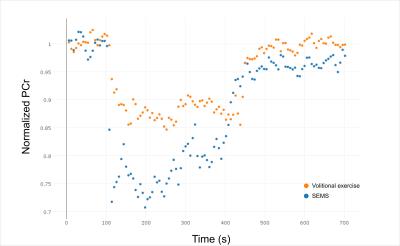
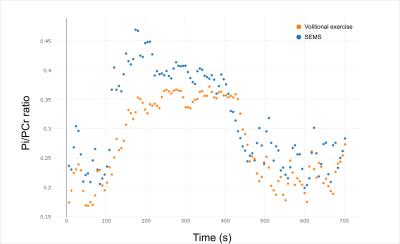
The preliminary experiments showed that triggering was necessary to obtain analyzable spectra (fig. 2, 3) to avoid electromagnetic interference. In the exercise sessions, the one with the lower EMS intensity of 20mA did not show an appreciable difference with respect to volitional exercise. However, the SEMS session at 25mA showed a clear difference in the PCr depletion curve with a higher PCr depletion for SEMS (fig. 4) and a higher Pi/PCr ratio (fig. 5). In this session, the Qmax was 0.57 for SEMS and 0.33 for volitional exercise and PCr depletion was 19% for SEMS and 13% for volitional exercise. The PCr recovery rate was 18.6s-1 for SEMS and 20.4s-1 for volitional exercise. No appreciable difference in pH could be measured.Discussion
The acquisition of 31P spectra during exercise with superimposed electrical stimulation is challenging, due to the necessity of synchronizing the stimulation, the volitional contraction and the acquisition. However, preliminary results indicate that for the same workload (in terms of antagonizing force and frequency of contractions), the additional EMS can help increase the metabolic response of the muscle, possibly thanks due to the preferential recruitment of type II muscle fibers1. In this work, we presented a solution that allows the implementation of this method with little additional cost and complexity with respect to a conventional 31P experiment. The demonstrated requirement of a minimum threshold of stimulation is in line with previous reports of synchronous EMS-MRI6.Conclusion
Superimposed EMS with volitional exercise can be an effective tool for the study of muscle metabolism in individuals that have difficulties in performing exercise, thanks to the increased metabolic response for equal workloads.Acknowledgements
This work was supported by the Swiss Foundation for the Research on Muscle Diseases (FSRMM) and the Lorenzo Piaggio foundation.References
1. Vanderthommen M, Duteil S, Wary C, Raynaud JS, Leroy-Willig A, Crielaard JM, Carlier PG. A comparison of voluntary and electrically induced contractions by interleaved 1H- and 31P-NMRS in humans. J Appl Physiol 2003;94:1012–1024.
2. Wahl P, Schaerk J, Achtzehn S, Kleinöder H, Bloch W, Mester J. Physiological responses and perceived exertion during cycling with superimposed electromyostimulation. J. Strength Cond. Res. Natl. Strength Cond. Assoc. 2012;26:2383–2388.
3. Wahl P, Hein M, Achtzehn S, Bloch W, Mester J. Acute effects of superimposed electromyostimulation during cycling on myokines and markers of muscle damage. J. Musculoskelet. Neuronal Interact. 2015;15:53–59.
4. Kemp GJ, Meyerspeer M, Moser E. Absolute quantification of phosphorus metabolite concentrations in human muscle in vivo by 31P MRS: a quantitative review. NMR Biomed. 2007;20:555–565.
5. Kemp GJ, Taylor DJ, Radda GK. Control of phosphocreatine resynthesis during recovery from exercise in human skeletal muscle. NMR Biomed. 1993;6:66–72.
6. Deligianni X, Pansini M, Garcia M, Hirschmann A, Schmidt-Trucksäss A, Bieri O, Santini F. Synchronous MRI of muscle motion induced by electrical stimulation. Magn. Reson. Med. 2016. doi: 10.1002/mrm.26154.
Figures