2933
Measurement of human cardiac intracellular pH in vivo using long TR 31P-MRS with adiabatic excitation at 7T1Oxford Centre for Clinical MR Research (OCMR), RDM Cardiovascular Medicine, University of Oxford, Oxford, United Kingdom, 2Department of Imaging Methods, Institute of Measurement Science, Slovak Academy of Sciences, Bratislava, Slovakia
Synopsis
Determination of human cardiac intracellular pH using 31P-MRS is challenging as the resonance frequency of Pi is concealed by a close resonating 2,3-DPG signal originating from blood. Common short TR and low-flip angle scan used for cardiac 31P-MRS increase the effective SNR/time, but can additionally suppress the Pi signal intensity. We have investigated the feasibility of detecting cardiac Pi and calculating intracellular pH of human heart using long TR 3D-CSI examination with adiabatic excitation at 7T. Comparison to short TR acquisition was performed using interleaved TR measurements. We report robust and repeatable detection of Pi signal in 100% of subjects.
Introduction:
Phosphorus MR spectroscopy (31P-MRS) is a valuable technique for non-invasive measurement of high-energy metabolites, e.g. adenosine triphosphate (ATP) and phosphocreatine (PCr), that allows assessment of tissue energy metabolism in vivo. The interest in 31P-MRS is great in cardiovascular medicine, as the PCr/ATP ratio in the heart changes in most major disease states1,2. Nevertheless, the full potential of cardiac 31P-MRS is not robustly accessible, i.e. the determination of cardiac intracellular pH is hindered by the dominant signal of 2,3-diphosphoglycerate (2,3-DPG), originating from the blood, which obscures the inorganic phosphate (Pi) resonance frequency. Several groups reported cardiac intracellular pH measured by 1H-decoupled 31P-MRS at 1.5T in the 1990s3,4. However, it would be practical to measure the cardiac intracellular pH at ultra-high fields (e.g., 7T), which are beneficial for cardiac 31P-MRS5. The enhanced spectral resolution at 7T should improve the detectability of cardiac Pi, but typical 31P cardiac spectra are acquired using short TR and low flip-angle to maximize the SNR/time, causing strong partial saturation of cardiac metabolites, e.g., Pi, whilst the blood pool is replenished, thus causing less pronounced saturation of the 2,3-DPG signal.The aim of this study was to examine the feasibility of robust cardiac intracellular pH measurement in vivo utilizing long TR with high flip-angle adiabatic excitation at 7T.
Methods:
All measurements were performed on a 7T MR system (Siemens) equipped with a custom dual-tuned RF-coil, comprising a quadrature 31P coil (two 15cm loops, with overlap decoupling) and a single 1H loop (10cm in diameter)6 for localization and shimming.In total, eight healthy volunteers (7M/1F) were recruited in compliance with local regulations and were examined with the coil positioned over their heart. First, one volunteer was examined using two consecutive 3D UTE-CSI8 acquisitions (matrix size of 8x16x8 and a FOV of 240x240x200mm3) with short and long TR (1s and 5s, respectively). The NA was matched for equal acquisition time, giving 41 averages for short and 5 averages for long TR measurement. An amplitude-modulated excitation pulse applied in previous 7T studies was used5.
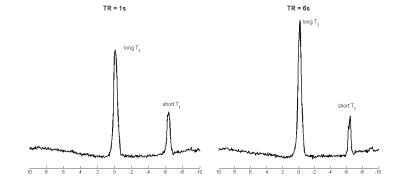
To be able to use the recently proposed AHP pulse7 at short TR, the 3D UTE-CSI8 sequence was modified to allow an interleaved acquisition with two different TRs9. The interleaved-TR sequence was first tested in a phantom (two phosphate solutions with different T1s), and then used in three of the volunteers for direct comparison of Pi detectability. The two selected TRs were 1s and 6s (effective TR for recently proposed AHP excitation at 7T)7. The excitation frequency of the AHP pulse for the in vivo measurements was centred at 2,3-DPG signal, i.e. 700 Hz from PCr.
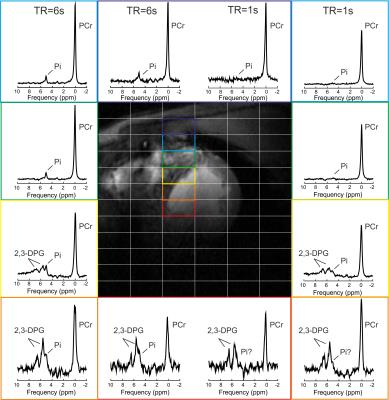
Finally, all recruited volunteers underwent 31P-MRS examinations using the previously proposed protocol for cardiac 31P-MRS using interleaved AHP excitation at 7T7, with effective TR of 6s. The experiment was performed twice to assess the repeatability of the pH determination. The in vivo data were fitted using a Matlab implementation of AMARES10 and corrected for T1 relaxation5. The pH of a mid-ventricular septum was compared between the two measurements by the Bland-Altman analysis of agreement11.
Results and Discussion:
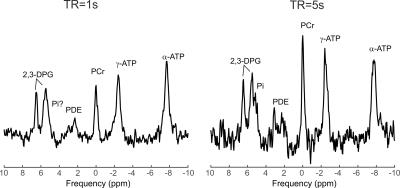
Figure 1 depicts the spectra from using two consecutive acquisitions with short and long TR using amplitude-modulated excitation pulse. The Pi signal demonstrates increased amplitude in the long-TR dataset, making it readily visible in vivo.Figure 2 provides the results of the phantom tests of the interleaved TR sequence, showing equal signal for short T1 solution (right) and saturation effect in the short TR dataset on the peak with long T1 (left), as expected. Figure 3 depicts the in vivo spectra from the comparison of short and long TR using adiabatic excitation and interleaved TR. Increased detectability of the Pi peak when using long TR with adiabatic excitation is visible in all voxels, not only in the mid-ventricular septum.
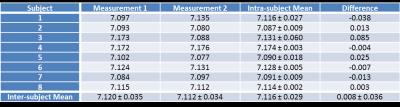
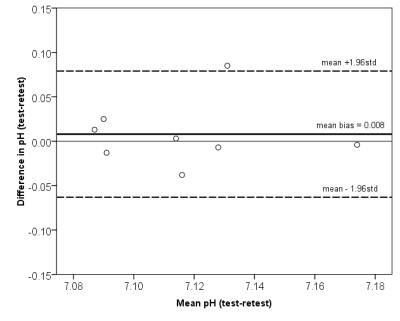
The Pi signal was detected in all measured volunteers and the calculated cardiac intracellular pH values from both UTE-CSI acquisitions are denoted in Table 1. The mean cardiac pH was 7.12±0.03, what is in good agreement with previous studies at lower field strengths (mean 7.08-7.15)3,4,12. Comparing the two repeated acquisitions, the mean bias given by the Bland-Altman analysis of agreement (Figure 5) was only 0.008 with the repeatability coefficient of 0.071. Thus, the AHP protocol with long TR at 7T allows repeatable and robust calculation of cardiac intracellular pH.
Conclusion:
This proof-of-concept study demonstrates that long TR acquisition at 7T combined with AHP excitation enables robust detection of cardiac Pi (100% of subjects), and thus, quantification of cardiac intracellular pH in vivoAcknowledgements
This work was funded by a Sir Henry Dale Fellowship from the Wellcome Trust and the Royal Society (grant #098436/Z/12/Z).References
1. Bottomley PA, Herfkens RJ, Smith LS, et al. Altered phosphate metabolism in myocardial infarction: P-31 MR spectroscopy, Radiology 1987;165:703-7
2. Neubauer S, Krahe T, Schindler R, et al. 31P magnetic resonance spectroscopy in dilated cardiomyopathy and coronary artery disease. Altered cardiac high-energy phosphate metabolism in heart failure, Circulation 1992;86:1810-8
3. de Roos A, Doornbos J, Luyten PR, et al. Cardiac metabolism in patients with dilated and hypertrophic cardio-myopathy: Assessment with proton-decoupled P-31 MR spectroscopy, J Magn Reson Imaging 1992;2:711-9
4. Jung WI, Sieverding L, Breuer J, et al. 31P NMR spectroscopy detects metabolic abnormalities in asymptomatic patients with hypertrophic cardiomyopathy, Circulation 1998;97:2536-42
5. Rodgers CT, Clarke WT, Snyder C, et al. Human cardiac 31P magnetic resonance spectroscopy at 7 Tesla, Magn Reson Med 2014;72(2):304-15
6. Schaller B, Paritmongkol W, Magill A, et al. Quadrature 31P and Single 1H Dual-Tune Coil for Cardiac 31P-MRS at 7T, Proc. ISMRM 2016;24:4006
7. Valkovic
L, Clarke WT, Purvis LAB, et al. Evaluation of PCr/ATP ratio in the human heart at 7T using adiabatic excitation, Proc ESMRMB 2016;33:217
8. Robson MD, Tyler DJ, Neubauer S. Ultrashort TE chemical shift imaging (UTE-CSI), Magn Reson Med 2005; 53(2):267-74.
9. Yarnykh VL. Actual flip-angle imaging in the pulsed steady state: a method for rapid three-dimensional mapping of the transmitted radiofrequency field, Magn Reson Med 2007;57:192-200
10.Purvis LAB, et al. Proc.
ISMRM 2014;22:2885
11.Altman DG & Bland JM.
Measurement in Medicine: the Analysis of Method
Comparison Studies, The Statistician 1983;32(3):307-17
12.Blamire AM, Rajagopalan B, Radda GK. Measurement of myocardial pH by saturation transfer in man, Magn Reson Med 1999;41:198-203
Figures