2924
In Vivo 3T Clinical Magnetic Resonance Imaging with a Biologically Specific Contrast Agent in Prostate Cancer: A Nude Mouse Model1Lakehead University, Thunder Bay, ON, Canada, 2Thunder Bay Regional Research Institute, Thunder Bay, ON, Canada, 3Thunder Bay Regional Health Science Center, Thunder Bay, ON, Canada
Synopsis
In this study we characterized in vivo a functional superparagmagnetic iron-oxide magnetic resonance contrast agent that effects the T2 relaxation time in MRI. The agent was developed by conjugating Molday Ion Carboxyl-6 (MIC6), with a de-immunized mouse monoclonal antibody (muJ591) targeting prostate-specific membrane antigen (PSMA). We propose this functional contrast agent as a non-invasive method to detect prostate cancer cells that are PSMA positive to provide increased differentiability from surrounding tissues for treatment. PSMA-positive prostate tumours were induced into 20 immunocompromised mice. The functional contrast agent was injected into 14 mice leaving 6 mice as controls. MR imaging was performed on a clinical 3T scanner using different parameters on a MESE sequence to obtain T2 relaxation time values. Tumour size, signal intensity, and T2 relaxation time were obtained pre and post injection and were found to have a lower value for treated mice compared to controls. ICP confirmed the increased level of elemental iron in treated mice tumours compared to controls. H&E staining showed healthy morphology of all tissues collected. The reduction in T2 relaxation time for the functional contrast agent, combined with its specificity against PSMA suggest its potential as a biologically-specific MR contrast agent.
Purpose
In this study we characterized in vivo a functional superparagmagnetic iron-oxide magnetic resonance contrast agent that effects the T2 relaxation time in MRI.Methods
The contrast agent was developed by conjugating Molday Ion Carboxyl-6 (MIC6), with a de-immunized mouse monoclonal antibody (muJ591) targeting prostate-specific membrane antigen (PSMA).1 We propose this functional contrast agent as a non-invasive method to detect prostate cancer cells that are PSMA positive to provide increased differentiability from surrounding tissues for treatment. PSMA-positive prostate tumours were induced into 20 immunocompromised mice. MR imaging was performed on a clinical 3T MRI with an 8-channel wrist RF coil ,where tumour size, signal intensity, and T2 relaxation time were obtained. Particle size analysis was performed using a Zetasizer Nano ZS instrument. A treatment group consisting of eleven mice was injected with the contrast agent at an average iron concentration of 60 μg/μL and 3 control mice were injected with saline as a control (6 did not grow suitable tumours). Multi-Echo Spin-Echo (MESE) sequences were used to obtain the T2 relaxation time before injection, immediately after, 10 min, 20 min, 30 min, 40 min, 1 day, 2 days and 3 days post injection. Mice were then euthanized and organs harvested. Inductively coupled plasma (ICP) was used to determine the elemental iron contents of the tissue samples and H&E staining was used to assess the viability of tissues on injected animals.Results
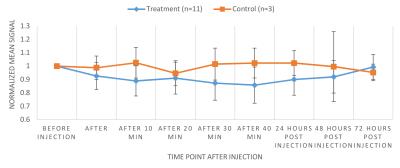
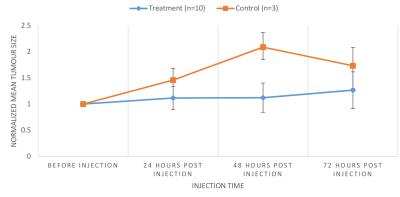
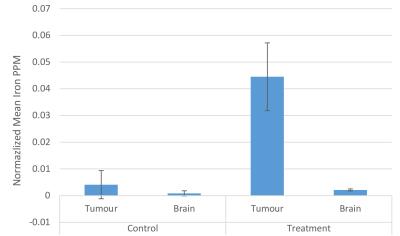
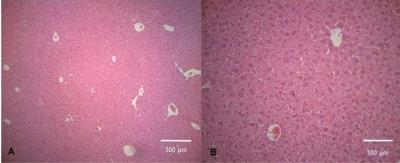
The zeta potential of antibody labeled complexes in different solutions was calculated to assess the stability in different buffers for injection. Sodium bicarbonate as a buffer had the highest zeta potential of 11.00 ± 2.56 mV compared to 1.44 ± 2.88 mV for saline-buffer. MR Signal from T2-weighted images decreased overtime for animals in the treatment group compared to control group however no signal reduction is detected 3 days post injection for either group (Fig 1). T2 relaxation time maps for a treatment and a control mouse are shown in Fig 2. A decrease in T2 relaxation time post injection can be observed for the treatment mouse where none is observed for the control. The T2 relaxation time value dropped by 28% 30 min after injection. No reduction in T2 relaxation time can be seen 3 days post injection suggesting that the amount of MIC6A was below the detection threshold of MRI after 3 days. The treatment group displayed a significant reduction in the area of the tumour central cross-section area 2 days after injection (Fig 3, p=0.0006). At this time point, the mean tumour area of the treatment group increased 12 ± 2 8% whereas the control group increased 209 ± 24% ICP-AES results showed a significant increase of iron levels between the treatment and the control group (Fig 4) as assessed by a two-tailed student t-test with equal variance (p=0.007). Brain tissue was used as control and did not show any change in iron levels. H&E was performed on all tissue samples collected and healthy morphology was confirmed by a pathologist (Fig 5).Discussion
We were able to test in vivo a functional contrast agent developed from muJ591 and MIC6. It was possible to detect the contrast agent immediately after injection and within the first 24 hours post-injection. The presence of the antibody could be inferred from the effect it had on tumour size 2 days post injection since it has been reported the antibody has a therapeutic effect.2 The high sensitivity of ICP allowed us to confirm an increase of iron levels in treated tumours compared to controls. However, we could not conclusively detect the presence of the contrast agent in the treated group 3 days post injection using MRI.Conclusion
In conclusion, we demonstrated the use of the developed conjugate muJ591:MIC6, which can specifically detect PSMA-expressing prostate cancer cells using a 3T Clinical MRI. Both T2 relaxation time and signal intensity was reduced after injection of the conjugate, aiding in the potential targeting and diagnosis of prostate cancer. Our findings suggest that muJ591:MIC6 could be a promising contrast agent that also showed signs of a therapeutic effect in reducing the size of prostate tumours.Acknowledgements
No acknowledgement found.References
1. Bates D, Abraham S, Campbell M, Zehbe I, Curiel L. Development and Characterization of an Antibody-Labeled Super-Paramagnetic Iron Oxide Contrast Agent Targeting Prostate Cancer Cells for Magnetic Resonance Imaging. Furlan R, editor. PLoS ONE. 2014;9: e97220. doi:10.1371/journal.pone.0097220
2. Bander NH, Trabulsi EJ, Kostakoglu L, Yao D, Vallabhajosula S, Smith-Jones P, et al. Targeting Metastatic Prostate Cancer With Radiolabeled Monoclonal Antibody J591 to the Extracellular Domain of Prostate Specific Membrane Antigen. J Urol. 2003;170: 1717–1721. doi:10.1097/01.ju.0000091655.77601.0c
Figures