2921
MRI biomarkers for PEGPH20-enhanced treatment of pancreatic ductal adenocarcinoma1Radiology, University of Washington, Seattle, WA, United States, 2Clinical Research Division, Fred Hutchinson Cancer Research Center, Seattle, WA, 3Medical Devices Research, National Inststitute of Health, Bethesda, MD, 4Department of Biomedical Engineering, University of Minnesota, Minneapolis, MN
Synopsis
Pancreatic cancer is a devastating disease with poor prognosis. Pancreatic tumor therapy has been ineffective in part because pancreatic tumors have high interstitial fluid pressure (IFP), driven by high hyaluronan concentration, that inhibits penetration of drugs into the tumor. We performed multi-parametric MRI at high resolution to non-invasively assess tumor response in a KPC mouse model to pegylated recombinant hyaluronidase in isolation as well as combined with Gemcitabine. T1 and T2 relaxation as well as diffusion, and 3 dimensional volume measurements were used to characterize the tumors. MR measurements were compared with invasive IFP measurements and histopathological results.
Purpose
Pancreatic cancer is the fourth leading cause of cancer-related deaths in the United States, with 5 year survival rate <5%.1, 2 Unique features of pancreatic ductal adenocarcinomas (PDAs) include a robust fibroinflammatory stroma as well as a dense extracellular matrix that accumulates water molecules in a poorly mobile, gel-fluid phase.3 In combination, these features result in a high tumor interstitial fluid pressure (IFP; ~99mmHg versus 10.4 in normal pancreas) that collapses tumor vasculature and impedes therapeutic drug delivery.4 Hyaluronan (HA; a naturally occurring glycosaminoglycan) is produced by PDA tumor cells, is often present at exceedingly high concentrations in the tumor interstitium, and its presence correlates with high tumor IFP.3, 5 In a genetic mouse model of PDA (KPC) that is known to recapitulate the progression of the human disease, intravenous delivery of pegylated recombinant hyaluronidase (PEGPH20 or “PEG”) has: 1) depleted tumor HA, 2) decreased IFP, 3) improved delivery of Gemcitabine (Gem), and 4) significantly prolonged survival.3, 4 A recent phase 1B clinical trial of PEG/Gem in patients with stage IV PDA demonstrated good tolerability and doubled overall survival in patients with high HA tumors.6 In clinical trials and in clinical practice, both the time and invasiveness required to assess treatment efficacy are critical considerations for the majority of patients with PDA who have rapidly progressive, non-surgical disease in a highly sensitive anatomic area. Our purpose is to implement non-invasive MRI methods to assess the degree of stromal depletion following PEGPH20 treatment, correlating our findings with tumor and treatment-specific IFP data previously published by our group, as well as histopathology.Methods
Tumor bearing, genetically engineered KPC mice (average age 5 months) were used in combination with multi-parametric MRI at 14T (Paravision 5.1 software, Bruker Corp, Billerica, MA) to assess tumor response to PEGPH20 alone, and combined with Gem. T1 and T2 relaxation as well as ADC measurements were acquired for 7 animals pre-PEGPH20, and 24 hours following intravenous treatment with 15 mg/kg (day 1). 5 of these animals were sacrificed immediately following day 1-post-treatment imaging. The remaining 2 animals received Gem on day 2, then similar PEG/Gem treatments on days 8/9 and 15/16, mirroring the clinical regimen, and were finally imaged and euthanized on day 24. Tumor volumes were measured in 1 of the 2 PEG/Gem animals at the 3 imaging time points. Histopathologic analysis at day 24 included H&E and markers of cellular proliferation (ki67) and apoptosis (CC3).Results
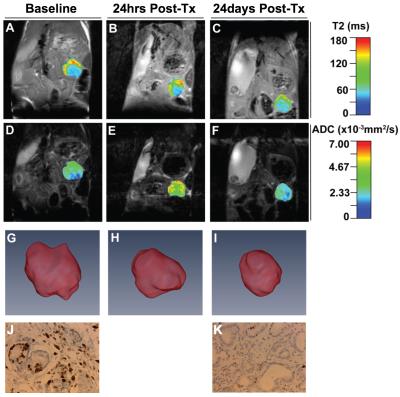
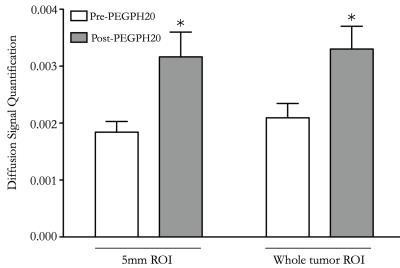
Pre-treatment, day-1 post-PEGPH20, and day 24 post-PEG/Gem regimen quantitative MR images are shown in Figure 1. On day 1 post-PEGPH20, both 5mm ROI and whole-tumor ROI analysis demonstrated significant increase in diffusion, as depicted in Figure 2, similar in time course to previously published trends in IFP change following PEG treatment.3 ADC map reverted to baseline on day 24 post-treatment, after 7 days had elapsed since this last PEG treatment. T2 maps demonstrated a trend toward increased signal intensity post-treatment on day 1, but this difference was not statistically significant. Tumor volumes were 87, 78 and 63 microL at pre-treatment, day 1 post-treatment, and day 24 post-treatment, respectively (Figure 1). Ki67 and CC3 stains showed significantly decreased (p = 0.01), and increased (p = 0.01) number of positive cells per field, respectively, 24 days post-treatment versus control (Figure 1), similar to previously published work.4Discussion
IFP reduction and fluid mobilization in tumors induced by PEGPH20 is most likely responsible for: 1) the significant increase in diffusion, 2) trend toward increased T2 map signal intensity and 3) diminished tumor volume observed on day-1 post treatment imaging. The progressive tumor volume reduction observed on day 24 post PEG/Gem regimen is most likely due to improved Gem penetration to the tumor and associated tumor cell apoptosis, as previously demonstrated by our group and reflected in our histopathologic analysis (Figure 1).4Conclusions
Multi-parametric MRI utilizing T2, ADC and 3D volume measurements can identify and monitor PEGPH20-enhanced treatment responses in the KPC mouse model of PDA. A similar imaging protocol could be employed in planned phase 2 clinical trials of this treatment regimen to provide non-invasive, rapid determination of tumor response.Acknowledgements
No acknowledgement found.References
1. Sun H, Ma H, Hong G, Sun H, Wang J. Survival improvement in patients with pancreatic cancer by decade: a period analysis of the SEER database, 1981-2010. Sci Rep 2014;4:6747.
2. Ades, T. et al. Cancer Facts and Figures 2014. American Cancer Society, Inc. 2014; http://www.cancer.org.
3. DuFort CC, DelGiorno KE, Carlson MA, Osgood RJ, Zhao C, Huang Z, et al. Interstitial Pressure in Pancreatic Ductal Adenocarcinoma Is Dominated by a Gel-Fluid Phase. Biophys J 2016;110(9):2106-19.
4. Provenzano PP, Cuevas C, Chang AE, Goel VK, Von Hoff DD, Hingorani SR. Enzymatic targeting of the stroma ablates physical barriers to treatment of pancreatic ductal adenocarcinoma. Cancer Cell 2012;21(3):418-29.
5. Fraser JR, Laurent TC, Laurent UB. Hyaluronan: its nature, distribution, functions and turnover. J Intern Med 1997;242(1):27-33.
6. Hingorani SR, Harris WP, Beck JT, Berdov BA, Wagner SA, Pshevlotsky EM, et al. Phase Ib Study of PEGylated Recombinant Human Hyaluronidase and Gemcitabine in Patients with Advanced Pancreatic Cancer. Clin Cancer Res 2016;22(12):2848-54.
Figures