2914
Non-gaussian IVIM imaging biomarkers can non-invasively predict the aggressiveness of small papillary thyroid cancers1Medical Physics, Memorial Sloan-Kettering Cancer Center, NEW YORK, NY, United States, 2Radiation Oncology, Washington University in St. Louis, St. Louis, MO, United States, 3Radiology, Memorial Sloan-Kettering Cancer Center, NEW YORK, NY, United States, 4Pathology, NYU Langone Medical Center, NEW YORK, NY, United States, 5Epidemiology and Biostatistics, Memorial Sloan-Kettering Cancer Center, NEW YORK, NY, United States, 6Pathology, Memorial Sloan-Kettering Cancer Center, NEW YORK, NY, United States, 7Surgery, Memorial Sloan-Kettering Cancer Center, NEW YORK, NY, United States, 8Medicine, Memorial Sloan-Kettering Cancer Center, NEW YORK, NY, United States
Synopsis
There is a need for non-invasive imaging to identify patients with aggressive tumors in papillary thyroid carcinoma (PTC). This study evaluates whether non-gaussian intravoxel incoherent motion (NG-IVIM) DW-MRI has the potential to stratify tumor aggressiveness in PTC. Twenty-four PTC patients underwent pretreatment NG-IVIM DW-MRI at 3T. The apparent diffusion coefficient (ADC), perfusion factor (f), diffusion (D),
Purpose
High-resolution neck ultrasonography (US) is now recommended and used in active surveillance for papillary thyroid carcinoma (PTC) patients (1-5). However, for PTCs with tumor size (1-2 cm), US has suboptimal sensitivity and specificity in the detection of aggressive features (6-8). Previous studies have shown that ADC (apparent diffusion coefficient) derived from DW-MRI was clinically relevant in assessing extrathyroidal extension in what appeared to be intrathyroidal papillary micro-carcinomas (tumor size <1cm) (9). In this study, a non-gaussian intravoxel incoherent motion (NG-IVIM) DW-MRI model developed in-house (10) was used to quantify diffusion and perfusion metrics to identify imaging biomarkers that preclude an active surveillance management approach for certain papillary thyroid cancers.Methods
Patients and Multi-b value DW-MRI data acquisition: Our institutional review board approved this retrospective study and issued a waiver of informed consent. Twenty-four patients with PTC (age: 27-78 years, M/F: 8/16) were referred for a multi-b value DW-MRI study by physicians at our institution. All patients underwent pretreatment MRI on a GE 3T scanner with a 24-channel neurovascular phased-array coil prior to surgery. Multi-b value DW-MRI acquisitions were performed using a SS-EPI sequence (TR = 4000 ms, TE = minimum [ms], and 3 orthogonal directions) with b values of 0, 20, 50, 80, 200, 300, 500, 800, 1000, 1500 s/mm2, 4-8 slices of 5 mm thickness covering the tumor, FOV=20~24 cm, and acquisition matrix =128 × 128. Multi-b value DW-MRI data analysis: The regions of interest (ROI) on the tumor were placed by an experienced neuroradiologist. The ADC was calculated using the conventional mono-exponential model. The perfusion factor (f), diffusion coefficient (D), pseudo diffusion coefficient (D*) and diffusion Kurtosis coefficient (K) were calculated using the non-gaussian intravoxel incoherent motion model (NG-IVIM) developed in-house (10). Histopathologic examination: All patients underwent surgery after the MRI exams. The surgical specimen was reviewed by an experienced pathologist. Tumor aggressiveness was evaluated for each surgical specimen using standard histopathologic features (9). Ultrasonography measurements: US examinations were performed according to standard protocol that included grayscale and color Doppler US assessment of the thyroid bed and cervical lymph nodes in all neck compartments. Tumor size was defined as the largest diameter among the 3 dimensions provided. Statistical analysis: ADC, D, f, D* and K values among 2 different groups (i.e., tumors with aggressive features, tumors without aggressive features) were statistically analyzed to determine whether they significantly differed by using a Non-parametric Mann-Whitney U test. The ROC curve was used to assess the discriminative specificity, sensitivity, and accuracy between PTCs with and without features of tumor aggressiveness. A p-value <0.05 was considered significant.Results
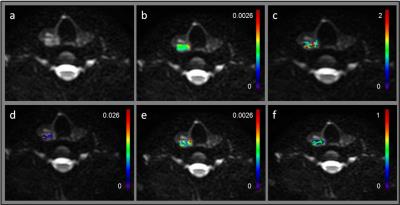
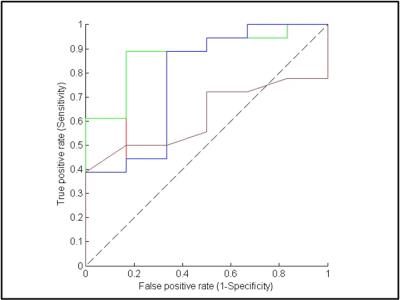
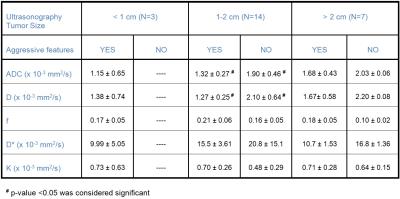
Figure 1 shows MRI from a representative PTC patient with tumor aggressive features (female; 28y; max. tumor diameter, 2.1 cm). Tumors with aggressive features had significantly lower ADC and D values and higher f values than that of the tumor without tumor aggressive features (p<0.05) while K and D* were not significantly different for the 2 groups (Table 1). Using ROC analysis, the cutoff values of ADC, D and f that discriminates between PTCs with and without aggressive features were determined [ADC = 1.87 · 10–3 mm2/s, D = 1.93 · 10–3 mm2/s and f = 0.14] with a sensitivity, specificity and ROC curve area of 88.89%, 88.83%, and 0.84, respectively, for ADC, 88.89%, 88.83%, and 0.88, respectively, for D, and 88.89%, 66.67% and 0.78, respectively, for f (Figure 2). Therefore, ADC, D, and f were found to exhibit promise to be surrogate biomarkers for aggressiveness in PTC patients. For the treating Physician, to recommend active surveillance for tumors measuring 1-2 cm in size by US is very difficult as no data is available to justify this in PTC patients. Our results show that out of the 24 patients, 14 patients were in the critical size range and the ADC, and D were significantly different (Table 1) and able to differentiate between the 2 tumor types (p<0.05). Also, that K, D* and f metrics were not significantly different in this cohort (p>0.05). Therefore, offering new imaging metrics in conjunction with clinical data for counseling patients who are better suited for active surveillance.Discussion and Conclusion
The magnitude of water diffusion reflects tissue micro-architecture while blood perfusion quantifies microcirculation in tumor tissues (10). Our study showed that ADC, and D may be used to distinguish tumors with and without aggressive features in tumor size ranging between 1-2 cm. ADC, and D exhibit their potential benefit in counseling PTC patients who may consider active surveillance and are not papillary micro-carcinomas (tumor size <1cm) on the onset.Acknowledgements
Supported by NCI/NIH grant (R21CA176660-01A1)References
1. Ito Y, Amino N, Miyauchi A. Thyroid ultrasonography. World journal of surgery. 2010;34(6):1171-80. doi: 10.1007/s00268-009-0211-3. PubMed PMID: 19823911. 2. Ito Y, Miyauchi A. Appropriate treatment for asymptomatic papillary microcarcinoma of the thyroid. Expert Opin Pharmaco. 2007;8(18):3205-15. doi: 10.1517/14656566.8.18.3205. PubMed PMID: WOS:000251666100010. 3. Ito Y, Masuoka H, Fukushima M, Inoue H, Kihara M, Tomoda C, et al. Excellent Prognosis of Patients with Solitary T1N0M0 Papillary Thyroid Carcinoma Who Underwent Thyroidectomy and Elective Lymph Node Dissection Without Radioiodine Therapy. World J Surg. 2010;34(6):1285-90. doi: 10.1007/s00268-009-0356-0. PubMed PMID: WOS:000277714300018. 4. Ito Y, Miyauchi A, Inoue H, Fukushima M, Kihara M, Higashiyama T, et al. An Observational Trial for Papillary Thyroid Microcarcinoma in Japanese Patients. World J Surg. 2010;34(1):28-35. doi: 10.1007/s00268-009-0303-0. PubMed PMID: WOS:000272906700005. 5. Wang LY, Roman BR, Palmer FL, Tuttle RM, Shaha AR, Shah JP, et al. Effectiveness of routine ultrasonographic surveillance of patients with low-risk papillary carcinoma of the thyroid. Surgery. 2016;159(5):1390-5. doi: 10.1016/j.surg.2015.11.018. PubMed PMID: 26747227; PubMed Central PMCID: PMC4991630. 6. Lee CY, Kim SJ, Ko KR, Chung KW, Lee JH. Predictive factors for extrathyroidal extension of papillary thyroid carcinoma based on preoperative sonography. Journal of ultrasound in medicine : official journal of the American Institute of Ultrasound in Medicine. 2014;33(2):231-8. doi: 10.7863/ultra.33.2.231. PubMed PMID: 24449725. 7. Gweon HM, Son EJ, Youk JH, Kim JA, Park CS. Preoperative assessment of extrathyroidal extension of papillary thyroid carcinoma: comparison of 2- and 3-dimensional sonography. Journal of ultrasound in medicine : official journal of the American Institute of Ultrasound in Medicine. 2014;33(5):819-25. doi: 10.7863/ultra.33.5.819. PubMed PMID: 24764337. 8. American Thyroid Association Guidelines Taskforce on Thyroid N, Differentiated Thyroid C, Cooper DS, Doherty GM, Haugen BR, Kloos RT, et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid : official journal of the American Thyroid Association. 2009;19(11):1167-214. doi: 10.1089/thy.2009.0110. PubMed PMID: 19860577. 9. Lu YG, Moreira AL, Hatzoglou V, Stambuk HE, Gonen M, Mazaheri Y, et al. Using Diffusion-Weighted MRI to Predict Aggressive Histological Features in Papillary Thyroid Carcinoma: A Novel Tool for Pre-Operative Risk Stratification in Thyroid Cancer. Thyroid : official journal of the American Thyroid Association. 2015;25(6):672-80. doi: 10.1089/thy.2014.0419. PubMed PMID: WOS:000363897500009. 10. Lu YG, Jansen JFA, Mazaheri Y, Stambuk HE, Koutcher JA, Shukla-Dave A. Extension of the intravoxel incoherent motion model to non-gaussian diffusion in head and neck cancer. J Magn Reson Imaging. 2012;36(5):1088-96. doi: 10.1002/jmri.23770. PubMed PMID: WOS:000310392800008.Figures