2908
Can diaphragm motion function as a surrogate for motion of esophageal tumors during treatment?1Department of Radiotherapy, University Medical Center Utrecht, Utrecht, Netherlands, 2Department of Surgery, University Medical Center Utrecht, Utrecht, Netherlands
Synopsis
Esophageal tumors show large motion in cranio-caudal direction, with a Peak-to-Peak (P-t-P) range of 2.7 to 24.5mm. In case the motion of the tumor could be followed during radiotherapy treatment, this would enable treatment margin reduction. It is researched whether the motion of the diaphragm is correlated with the breathing motion and drift we can detect in esophageal tumors since it could function as surrogate for tumor motion during treatment. A high correlation was found between both motion patterns and correction of the tumor motion using the diaphragm drift resulted on average in a reduction in P-t-P motion in all patients.
Purpose
Esophageal tumors show large motion in cranio-caudal direction (CC), with a Peak-to-Peak (P-t-P) range of 2.7 to 24.5mm1. In case the motion of the tumor could be followed during radiotherapy treatment, this would enable treatment margin reduction. The aim of this research is to investigate whether the motion of the diaphragm is correlated with the breathing motion and drift we can detect in esophageal tumors. As such, the diaphragm could function as a surrogate for esophageal tumor motion during treatment. Adaptive approaches could become feasible; e.g. use of a gating window to stop the treatment when the surrogate, and thus tumor, moves out of the selected window. Applying such a patient-tailored treatment approach could reduce treatment margins which are currently population-based.Methods
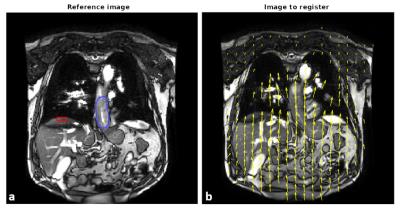
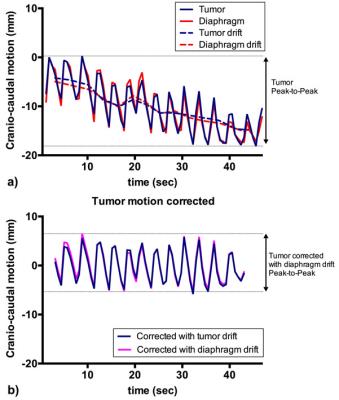
In total, 58 coronal cine MR scans were obtained from 5 patients whom were treated with neoadjuvant chemoradiotherapy (nCRT) for distal esophageal cancer. In this study, one MR scan was performed prior to nCRT, followed by 5 weekly MR scans during nCRT (in one patient only 4 weekly scans were acquired). Cine MR scans included 75 frames acquired at a rate of 1.6Hz, with a resolution of 2.01x2.01mm. The scan was acquired twice within one session, separated by circa 10 minutes. To estimate motion in the cine MR series an optical flow algorithm (RealTITracker2) was used to calculate motion fields (Fig. 1). The tumor was delineated manually, in which the mean motion for each frame was calculated in CC direction. Motion was also estimated in the diaphragm/liver border within a manually placed rectangle (Fig. 1a). An in-house tool was designed to find peaks and estimate drifts in the motion curves. Drift was defined as the change in the mean between consecutively found local maxima and minima. Correlation of the CC motion between diaphragm and tumor was calculated. P-t-P analysis was performed on tumor motion curves and tumor motion curves corrected for drift using the diaphragm drift (Fig. 2).Results
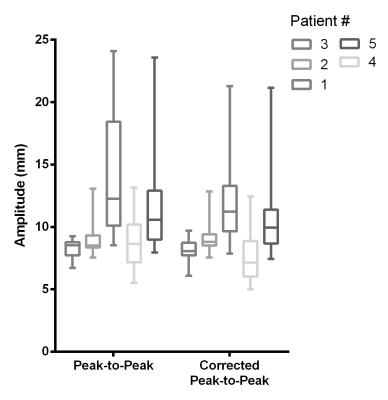
A strong Pearson’s correlation of r=0.971 was found while comparing CC motion in diaphragm and tumor, with a range of 0.849-0.996. The mean P-t-P tumor motion before and after correction for drift was 10.4 and 9.6mm respectively (p<0.01). However, for individual scan sessions the effect of drift could be much larger, as is exemplified in Fig. 2a. P-t-P amplitude for each patient before and after drift correction is shown in Fig. 3. Although the amplitude of the diaphragm motion was higher, mean P-t-P motion of 12.7mm, when the tumor motion showed a drift or sudden movement, this was also found in the diaphragm motion (Fig. 2&3).Conclusion
In this study it was found that diaphragm motion shows a strong correlation with esophageal tumor motion. Using the diaphragm motion for drift correction resulted on average in a reduction of the P-t-P range over all patients. This reduction can be used for adaptive treatment strategies, which reduce margins. For example, in case an MR-linac is taken in mind3, MR-based gating to compensate for respiratory motion and/or base-line shift (drifting) detection using the diaphragm as surrogate (ie. using a 1D navigator placed over the liver/lung interface) will be well feasible.Acknowledgements
No acknowledgement found.References
[1] Lever, F.M., Lips, I.M., Crijns, S.P.M., et al. (2013). Quantification of Esophageal Tumor Motion on Cine-Magnetic Resonance Imaging. International Journal of Radiation Oncology, Biology, Physics
[2] Zachiu C., Denis de Senneville B., Moonen C.T.W., et al. (2015) A framework for the correction of slow physiological drifts during MR-guided HIFU therapies: Proof of concept. Medical Physics
[3] Lagendijk J.J.W., Raaymakers B.W., Raaijmakers A.J.E., et al. (2008) MRI/linac integration. Radiotherapy and Oncology
Figures