2901
Combined analysis of ten DW-MRI studies demonstrates excellent repeatability of apparent diffusion coefficient estimates in extra-cranial soft-tissue tumours1Cancer Research UK Cancer Imaging Centre, The Institute of Cancer Research, London, United Kingdom, 2MRI Unit, The Royal Marsden NHS Foundation Trust, Sutton, United Kingdom, 3Cardiff University Brain Research Imaging Centre (CUBRIC), School of Psychology, Cardiff University, Cardiff, United Kingdom, 4Drug Development Unit, The Royal Marsden NHS Foundation Trust, Sutton, United Kingdom, 5Division of Clinical Studies, The Institute of Cancer Research, London, United Kingdom
Synopsis
Repeatability of ADC estimates from ten diffusion-weighted MRI studies in extra-cranial soft tissues (nine patient studies and one healthy volunteer study, with a total of 111 subjects) were analysed in combination using the Radiological Society of North America (RSNA) Quantitative Imaging Biomarkers Alliance (QIBA) framework for assessment of technical performance of imaging biomarkers, in order to investigate factors affecting ADC repeatability. Coefficient of variation was between 2 and 7% for all studies, with no marked differences between imaging protocols or study populations, and better repeatability in large tumours compared with smaller tumours, indicating that ADC is a robust imaging metric with excellent repeatability in extra-cranial soft-tissue tumours.
Background
Body diffusion-weighted-MRI (DW-MRI) is established as a qualitative and quantitative technique in oncology.1 Post-treatment changes in the apparent diffusion coefficient (ADC) have been shown to be indicative of response to chemotherapy and other treatments in a wide range of tumour types. The minimum significant change in ADC that can be detected after treatment is determined by the repeatability of the measurement. Double-baseline examinations are often incorporated into clinical trials in order to estimate ADC repeatability but conducting a second baseline examination is an additional burden on patients and resources. It is desirable to make use of repeatability estimates from previous studies, but this is hindered by variations in imaging protocols, tumour types, and patient cohorts, as well as variations in reporting ADC repeatability. The Radiological Society of North America (RSNA) Quantitative Imaging Biomarkers Alliance (QIBA) framework for assessment of technical performance of imaging biomarkers was developed to encourage consistency in assessment of technical performance, including repeatability, and is applied in this study.2Purpose
To assess ADC repeatability in a wide variety of DW-MRI studies of extra-cranial soft-tissue tumours and healthy organs, using methods proposed in the QIBA framework2, across a wide range of imaging protocols, tumour sites, tumour sizes, and patient populations.Methods
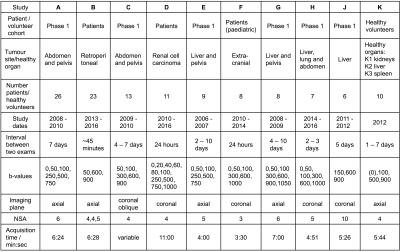
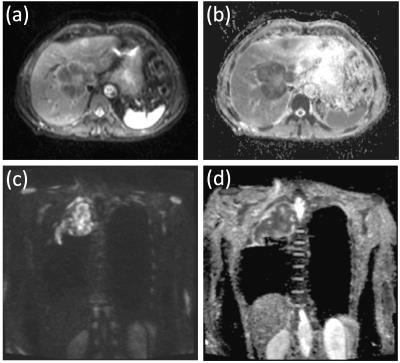
Nine patient studies and one healthy volunteer study were included in this analysis, with Research Ethics Committee approval and written consent. Study cohorts and imaging protocols are described in Figure 1; example images from two patients are shown in Figure 2. A total of 141 tumours/healthy organs were each imaged twice. For each tumour/organ, a volume of interest (VOI) was defined by outlining the whole area of the tumour/organ on at least 3 central slices. Median and mean ADC estimates (ADCmedian, ADCmean) were calculated for each VOI. ADC repeatability was assessed using the within-subject standard deviation (sw), within-subject coefficient of variation (CoV), limits of agreement (LoA), repeatability coefficient (RC), and intraclass correlation coefficient (ICC), with 95% confidence intervals estimated for all quantities.2
Levene’s test for equality of variance (Matlab2016a) was used to assess whether ADC repeatability differed between studies. Pearson’s linear correlation coefficient (Matlab2016a) was used to assess correlation between CoV and the year the study started, number of VOIs in the study, and the median volume of VOIs in the study.
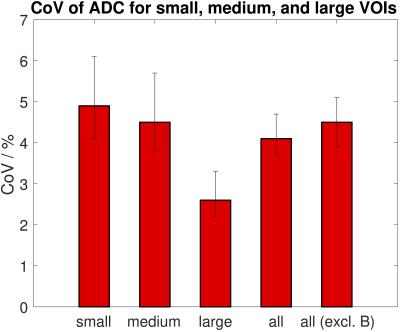
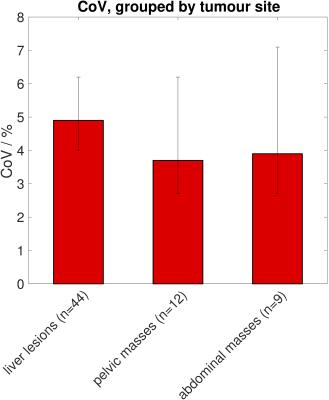
After analysis of each study separately, VOIs were further grouped into small, medium, and large volumes (Figure 4), and ADC repeatability assessed for the three groups. Tumours were also grouped by site (liver lesions, pelvic masses, abdominal masses) and ADC repeatability assessed for these sub-groups. Levene’s test was used to assess whether ADC repeatability differed between sizes and between sites.
Results
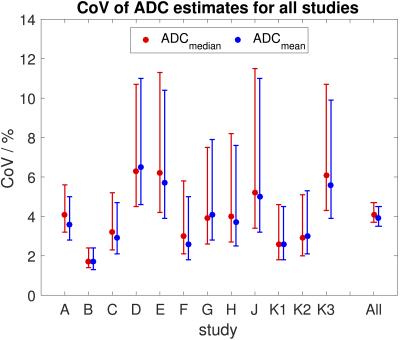
Repeatability of ADCmedian and ADCmean was good with CoVs between 1.7% and 6.5% for all studies (Figure 3). Repeatability of ADCmean was similar to repeatability of ADCmedian in each study (Figure 3). Levene’s test showed a significant difference in repeatability between studies (p<0.05), which did not persist after excluding the study with the lowest CoV (study B, which included many large tumours). No correlation was observed between the CoV and the year the study started nor the number of VOIs in each study. Weak correlation was observed between the CoV and the median volume of VOIs in the study (r=-0.5, p=0.1). There was a significant difference in ADC repeatability between small, medium, and large volumes with the smallest CoV for large VOIs (Figure 4, Levene’s test p<0.05). There was no difference in ADC repeatability between liver lesions, pelvic masses and abdominal masses (Figure 5, Levene’s test p=0.5). The upper 95% LoA was 20% or lower in all studies, with LoA of 12% for all VOIs analysed together.Discussion
The excellent repeatability in all of the studies included in this analysis demonstrates that ADC is a robust metric in clinical practice in oncology. Good repeatability was observed across a wide variety of imaging protocol variations, with no marked differences between study populations (phase 1 trial patients, paediatric patients, and healthy adult volunteers) or tumour sites. However, the fact that all studies were performed on single-vendor 1.5T scanners at the same institution remains a limitation. The significant difference in repeatability between small, medium and large tumours/organs, suggests that tumour size is an important factor in ADC repeatability. A post-treatment increase in ADC of 20% or more would be outside the LoA in all studies included in this analysis.Conclusions
In a single-centre setting, ADC is a robust imaging metric with excellent repeatability in extra-cranial soft-tissue tumours across a wide range of patient populations, tumour sites, sizes, and imaging protocol variations.Acknowledgements
We acknowledge CRUK and EPSRC support to the Cancer Imaging Centre at ICR and RMH in association with MRC and Department of Health C1060/A10334, C1060/A16464 and NHS funding to the NIHR Biomedical Research Centre and the Clinical Research Facility in Imaging. MOL is an Emeritus NIHR Senior Investigator.
For specific studies included in this work, we acknowledge funding from CRUK BIDD grants C7273/A12064 and C1353/A12762; CRUK and EPSRC Cancer Imaging Programme at the Children’s Cancer and Leukaemia Group (CCLG) in association with the MRC and Department of Health (England) (C7809/A10342); an Experimental Cancer Medicine Centre Network award (joint initiative, CRUK and UK Department of Health) grants C51/A7401 and C12540/A15573; EPSRC Platform Grant EP/H046526/1; Experimental Cancer Medicine Centre (ECMC) Network funding for support to early clinical trials; and the support of the National Institute for Health Research through the Cancer Research Network. Some of these studies were supported by AstraZeneca, Merck, Basilea, ArQule, and Genentech. MRO was funded by AstraZeneca.
References
1. Taouli B, Beer AJ, Chenevert T, et al. Diffusion-weighted imaging outside the brain: consensus statement from an ISMRM-sponsored workshop. J Magn Reson Imag 2016 DOI: 10.1002/jmri.25196.
2. Sullivan DC, Obuchowski NA, Kessler LG, et al. Metrology standard for quantitative imaging biomarkers. Radiology 2015;277(3):813-825.
Figures