2898
Probing infiltration of the normal brain by glioblastoma using magnetic resonance perfusion and permeability techniques1Glasgow Experimental MRI Centre, Institute of Neuroscience and Psychology, University of Glasgow, Glasgow, United Kingdom, 2Translational Radiation Biology, Institute of Cancer Sciences, University of Glasgow, Glasgow, United Kingdom
Synopsis
MRI techniques probing brain perfusion and blood-brain barrier (BBB) permeability were assessed in their ability to detect low tumour invasion in mouse glioblastoma models. A multiple boli Arterial Spin Labeling technique was optimised, achieving high SNR perfusion imaging. Diffusion-weighted arterial spin labelling allowed to separate fast motion vascular components of the signal from slow motion tissue components, providing with BBB permeability weighted maps. Evaluation was performed by comparison with conventional MRI and immunohistochemistry sections (HLA) cut in the MRI plane . Both perfusion weighted maps and BBB permeability weighted maps allowed to identify low tumour regions not detected with conventional MRI techniques.
Introduction
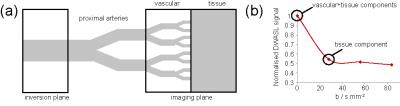
Glioblastoma (GBM) is one of the most aggressive and heterogeneous forms of brain cancer and is very difficult to treat. A major obstacle to successful treatment is the ability of GBM cells to invade healthy brain tissue. This makes complete removal of the tumour by surgery impossible, leading to high recurrence rates, and reduces the accuracy of target volume delineation for radiotherapy planning. While magnetic resonance imaging (MRI) is a valuable tool for clinicians treating GBM, conventional imaging techniques fail to detect regions of low tumour cell density that may be responsible for subsequent tumour recurrence. Invading tumour cells often progress along blood vessels and recent results indicate that even individual cells can disrupt the normal function of the blood brain barrier (BBB)1, providing an opportunity to detect tumour invasion at its earliest stages. Our research focuses on the development of MRI techniques that probe brain perfusion and BBB permeability, and their assessment as biomarkers for detecting low tumour infiltration regions in mouse GBM models. A high SNR multiple adiabatic boli Arterial Spin Labeling technique was optimised for rodent brain perfusion imaging2, and a method of diffusion-weighted arterial spin labelling (DWASL) was implemented in order to probe the molecular mobility of perfused blood water. Previous works3 suggest that the introduction of diffusion gradients into the ASL sequence allows minimising the contribution of fast motion vascular components of the signal, providing with perfusion images dominated by the slower motion tissue component (Fig.1). Using this model, the ratio of the tissue perfusion images obtained by DWASL to the overall perfusion ones obtained by ASL was expected to produce BBB permeability weighed maps. The resulting maps were compared with histological sections stained with (FITC)-dextrans to assess BBB disruption. The ability of perfusion and BBB permeability techniques to characterise tumour invasion was evaluated by comparison with standard MRI techniques and immunohistochemistry sections cut in the MRI plane.Methods
This study used nude mice (n=10) injected intra-cranially with human glioblastoma, presenting highly invasive tumour margins. In week 12 post-injection, the mice were scanned in vivo, injected with (FITC)-dextran and sacrificed. Imaging was performed on a Bruker 7T Biospec instrument with 72 cm resonance birdcage and a phase-array surface coil. Multiple Spin Multiple Echo, Inversion recovery, Pulsed Gradient Spin Echo and Arterial spin Labelling techniques were combined with RARE or EPI acquisition to provide with the following imaging modalities: T2 weighted (T2W), T2 value, Diffusion weighted (DW), Apparent Diffusion Coefficient (ADC), Fractional anisotropy(FA), perfusion weighted (ASL) and BBB permeability weighted (DWASL). Slice thickness was 1.5 mm and resolution varied according to the MRI technique used. Three different histology stains were used: Human leukocyte antigen (HLA) stain for tumour cell presence, 10 kDa and 70 kDa (FITC)-dextrans for respectively minor and major BBB disruption. Five evenly distributed histological slices were cut in the MRI plane and stacked to account for MRI slice thickness. Post processing of the data (with in-house developed MATLAB code) included: noise reduction, surface-coil sensitivity correction, re-gridding and MRI/Histology registration.Results and Discussion
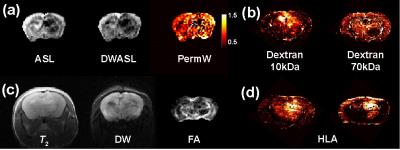
The permeability weighted maps obtained by the ratio of DWASL images to ASL images presented high values in the regions surrounding the tumour bulk (Fig. 2a). Histological slices stained with (FITC)-dextrans (Fig.2b) allowed identifying regions of BBB disruption (10kDa) and low perfusion (70kDa). Despite the fact that abnormal regions in the permeability weighted maps were found to be greater than the ones in dextran histology, several similarities were identified. The ability of perfusion and BBB permeability imaging techniques to characterise tumour invasion was evaluated by comparison with standard MRI techniques (Fig.2 c) and immunohistochemistry (HLA staining for human tumour cells) of brain sections cut in the MRI image plane (Fig.2d). Both ASL and DW-ASL techniques were shown to identify regions of low tumour cell density that were present on the HLA slices but not detected by conventional techniques. Furthermore, these techniques reveal additional qualitative properties of the tumour enabling a more detailed evaluation of tumour heterogeneity.Conclusion
Brain perfusion weighted maps (ASL) and BBB permeability weighted maps (ASL/DWASL) were in produced in mouse glioblastoma models. Their ability to probe tumour invasion was assessed by comparison with in-plane HLA histology. Both techniques were shown to detect low tumour regions not detected with conventional MRI techniques. Comparison with in-plane (FITC)-dextran histology allows to get a better understanding of the relation between the resulting images, brain perfusion and BBB disuption.Acknowledgements
This work was funded by The Brain Tumour Charity (grant ref. 26/160). The authors would also like to thank Mr James Mullin for his advice and fruitful discussions on animal setup and scanning protocols.References
1. Watkins, S.; Robel, S.; Kimbrough, I. F.; Robert, S. M.; Ellis-Davies, G.; Sontheimer, H., Disruption of astrocyte-vascular coupling and the blood-brain barrier by invading glioma cells. Nat Commun 2014, 5.
2. Valllatos, A.; Gilmour, L.; Chalmers, A. J.; Holmes, W. M., Multiple boli Arterial Spin Labelling for high signal-to-noise rodent brain perfusion imaging. Magn Reson Med 2016 (submitted)
3. Wang, J. J.; Alsop, D. C.; Song, H. K.; Maldjian, J. A.; Tang, K.; Salvucci, A. E.; Detre, J. A., Arterial transit time imaging with flow encoding arterial spin tagging (FEAST). Magn Reson Med 2003, 50 (3), 599-607.
Figures