2897
A population-based digital reference object (DRO) for optimizing dynamic susceptibility contrast (DSC) MRI methods for clinical trials1Translational Bioimaging, Barrow Neurological Institute, Phoenix, AZ, United States, 2Diagnostic Imaging, Rhode Island Hospital, Providence, RI, United States
Synopsis
The standardization of DSC-MRI has been confounded by a lack of consensus on DSC-MRI methodology for preventing potential rCBV inaccuracies, including the choice of acquisition protocols and post-processing algorithms. To address these issues, we developed a digital reference object (DRO) aimed at validating image acquisition and analysis methods for accurately measuring rCBV in glioblastomas. The DRO was developed using trained physiological and kinetic parameters derived from in vivo data, unique voxel-wise 3D tissue structures, and a validated MRI signal computational approach. The DRO’s ability to produce reliable signals was validated by comparison to separate cohort of patient data.
Introduction
In brain tumor DSC-MRI data, the acquired signals reflect a complex combination of T1, T2, and T2* changes that depend upon numerous features including contrast agent (CA) kinetic parameters, tissue physical and physiological parameters, and acquisition parameters. The standardization of DSC-MRI has been confounded by a lack of consensus on DSC-MRI methodology for preventing potential rCBV inaccuracies. To address these issues, we developed a DSC-MRI digital reference object (DRO) aimed at validating image acquisition and analysis methods for accurately measuring rCBV in glioblastomas.Methods
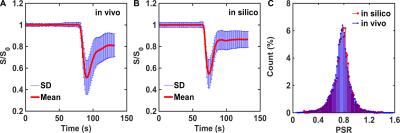
The DRO is driven by a validated computational strategy, termed the finite perturber finite difference method, which computes MRI signals for realistic 3D tissue structures and accounts for the static magnetic field strength, inter-compartment susceptibility differences, the water proton diffusion coefficient, and pulse sequence parameters.1 The two-compartment pharmacokinetic model described by Brix et al. 2 was used to simulate concentration-time profiles in plasma (Cp) and the EES (Ce). Inputs to the Brix model include CBF, CBV, CA transfer coefficient (Ktrans), volume fraction of the EES (ve), and the arterial input function (AIF). To ensure clinical relevancy, the paired CBV and CBF distributions were extracted from voxel-wise data from 23 glioblastoma patients (> 40,000 voxels). The voxel-wise distributions of Ktrans and ve were characterized using a retrospective analysis of a DCE-MRI dataset in 11 glioblastomas.3 Since DSC-MRI data yield relative measures of tumor CBV and CBF, their values were scaled using data obtained from dynamic CT perfusion imaging.4 The resulting distribution was used to define the vascular and cellular properties of the tissue structures modeled using ellipsoids (cells) packed around randomly oriented cylinders (vessels). In order to ensure that the DRO’s simulated DSC-MRI signals accurately represent the magnitude and distribution of CA-induced T1 and T2* changes observed across typical glioblastomas we used the voxel-wise patient data to train the DRO to identify the relevant structural features. Specifically, all computed signals, for equivalent pulse sequence parameters to those in the patient dataset, underwent a selection criteria based on their percent signal recovery (PSR) and the mean and standard deviation of the signals across the DRO. The DRO’s input tissue structure (e.g. cell size, shape) and physical parameters (pre-contrast T1) were systematically permutated until the distribution of PSR values and their mean and standard deviation across all the simulated signals agreed with those found in the patient data. This iterative process yielded a DRO consisting of 10,000 unique voxels. Figure 1A-B shows the agreement between the in vivo and in silico mean and standard deviation of the signal. The distribution of PSR values obtained from the training dataset and the DRO is shown in Figure 1C. A two-sample Kolmogorov-Smirnov test yielded a p-value of 0.69, indicating agreement between the two distributions.Results
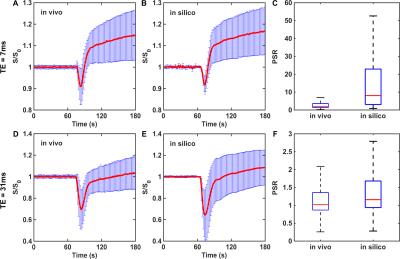
To validate the DRO’s ability to produce reliable signals for pulse sequences and contrast agent dosing schemes different from those in the training dataset, we compared simulated dual-echo signals to those found in an in vivo dual-echo DSC-MRI “validation” dataset acquired in a separate (and smaller) cohort of patients. To determine whether the DRO recapitulated the signal characteristics of the in vivo dual-echo data a correlation analysis was performed between the signals in the in vivo and DRO data. The range of PSR values found in the in vivo and DRO data were compared to ensure that the DRO captured the signal heterogeneity measured in the validation set for both echo times. Figure 2 compares simulated and in vivo dual-echo DSC-MRI data. The DRO was able to accurately recapitulate the TE = 7 ms and TE = 31 ms signal time courses. The PSR heterogeneity of the in vivo data was also fully reflected in the DRO. This indicates that the trained DRO can accurately model the underlying CA-induced T1 and T2* effects and the associated DSC-MRI signals for different sets of pulse sequence parameters and CA dosing schemes.Discussion/Conclusion
We have described the development of a DRO that recapitulates the DSC-MRI signal characteristics observed in human glioblastomas. Clinical relevance is ensured through the use of a training dataset. Furthermore, we validated the DRO’s ability to produce reliable signals for different CA dosing schemes and acquisition parameters. Currently, the proposed population-based DRO is being utilized to systematically investigate the influence of a range of acquisition and post-processing methods on CBV accuracy.Acknowledgements
NCI R01 CA158079References
1. Semmineh, N.B., et al., An efficient computational approach to characterize DSC-MRI signals arising from three-dimensional heterogeneous tissue structures. PLoS One, 2014. 9(1): p. e84764.
2. Brix, G., et al., Regional blood flow, capillary permeability, and compartmental volumes: measurement with dynamic CT--initial experience. Radiology, 1999. 210(1): p. 269-76.
3. Paulson, E.S., D.E. Prah, and K.M. Schmainda, Spiral Perfusion Imaging with Consecutive Echoes (SPICE) for the Simultaneous Mapping of DSC- and DCE-MRI Parameters in Brain Tumor Patients: Theory and Initial Feasibility. Tomography, 2016 (in press).
4. Schramm, P., et al., Dynamic CT perfusion imaging of intra-axial brain tumours: differentiation of high grade gliomas from primary CNS lymphomas. Eur Radiol, 2010. 20(10): p. 2482-90.
Figures