2894
Evaluation of DCE-MRI Exam with High-Temporal Resolution for Breast Cancer Diagnosis in a Biopsy Cohort1Center for Advanced Imaging Innovation and Research, Radiology, New York University School of Medicine, New York, NY, United States
Synopsis
The purpose of this study was to assess the feasibility of using GRASP DCE-MRI for contrast kinetic analysis to determine lesion malignancy. This IRB-approved retrospective study included 73 women who underwent MRI-guided biopsy scans. The plasma flow in the malignant group was significantly higher than that of the benign group with the area under the curve of 0.77. The results in this study successfully demonstrate that the GRASP DCE-MRI method can be used to acquire adequate high-temporal and high-spatial resolution images of the breast for contrast kinetic analysis.
PURPOSE
Dynamic contrast-enhanced (DCE) MRI is a highly sensitivity tool for breast cancer screening and is currently recommended as an annual screening exam by the American Cancer Society in high-risk patients.1 However, DCE-MRI suffers from low and variable specificity (26-97%).2-4 The clinical dilemma results from the overlap of the morphologic and kinetic characteristics between benign and malignant lesions5-7, which often leads to MRI-guided biopsies. Model-based contrast kinetic analysis of contrast agent uptake curves of suspicious lesions has been applied to temporal DCE-MRI data in an attempt to improve its diagnostic accuracy.8,9 But it remains challenging to acquire DCE-MRI data with a high spatial and high temporal resolution for clinical routine use. Recently, Golden-angle Radial Sparse Parallel (GRASP) MRI has been introduced to address this issue.8,10 The purpose of this study is to assess the feasibility of using GRASP DCE-MRI for contrast kinetic analysis to determine lesion malignancy, particularly in an MRI-guided biopsy cohort.METHODS
Data acquisition: This IRB-approved retrospective study included 73 women who underwent MRI-guided biopsy scans with DCE-MRI exam using a radial stack-of-stars 3D spoiled gradient echo pulse sequence with golden-angle spoke ordering. All scans were performed on a whole-body 3T scanner (MAGNETOM TimTrio, Siemens Healthcare, Erlangen, Germany) equipped with a seven element breast coil array (InVivo, FL). It included 56 women with pathology-proven benign lesions and 17 women with pathology-proven malignant lesions. Mild breast compression was used as in routine MRI-guided biopsy scans. Other imaging parameters were as follows: sagittal slab orientation, FOV=280 x 280 x 144 mm3, FA = 12 degrees, TE/TR = 1.47/3.6 ms, and BW = 710 Hz/pixel. A total of 2280 spokes were acquired for each of the 35 partitions during free breathing to cover one breast planned for biopsy. The reconstructed image matrix size per frame was 256x256x72 with zero padding along the slice direction. Two-fold readout oversampling (512 sample points/spoke) was used to minimize spurious aliasing along each spoke. The total acquisition time was 5 min 40 s. After baseline acquisition of 57 s, a single dose of Gd-DTPA (Magnevist, Bayer Healthcare, Leverkusen, Germany) at 0.1 mM/kg body weight was injected at 2 ml/sec into an antecubital vein while the scan continued for another 4 min 43 s. The GRASP image reconstruction method was used to generate dynamic 3D images with temporal resolution of 5 s/frame.
Data analysis: Regions of interest (ROI) for the lesions were manually selected to draw one ROI per lesion, after review of the MRI images and associated reports (Figure 1a and 1b). Three to five ROIs per subject were also selected to measure contrast kinetic parameters in the background parenchyma (Figure 1c and 1d). The images and time-intensity curves shown in Figure 1 demonstrate the advantages of using the high-temporal and high-spatial resolution images of GRASP DCE-MRI. For contrast kinetic model analysis of the lesion curves, we used the two-compartment exchange model (TCM)11 as shown in Figure 2a. The time-intensity curves of background parenchymal enhancement (BPE) typically showed slow and persistent enhancement, which is not adequate to estimate the extracellular volume fraction. Hence, we used a modified version of TCM model as shown in Figure 2b, referred to as two-compartment leakage model (TLM). The model parameter estimation was performed using in-house software developed in IDL (Excelis VIS, Boulder, CO).
RESULTS
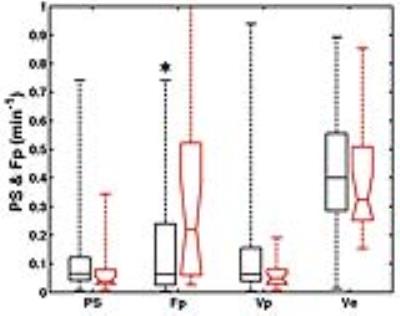
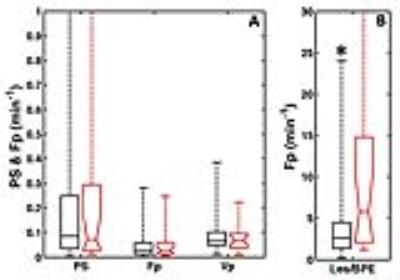
Figure 3 shows a summary of the TCM parameters of women with and without malignant lesions. The plasma flow in the malignant group was significantly (p< 0.05) higher than that of the benign group. The receiver-operating characteristic (ROC) analysis showed that the Fp parameter has an area under the ROC curve (AUC) of 0.77 with 88% sensitivity and 41% specificity, using the cutoff value of 0.32 min-1. Figure 4 shows the TLM parameters of BPE ROIs. There was no significant difference in any of the TLM parameters. The ratio of lesion flow to BPE flow (Figure 4b) was significantly (p< 0.05) difference between the two groups, with 82% sensitivity, 53% specificity, and AUC=0.75.DISCUSSION & CONCLUSION
The results in this study successfully demonstrate that the GRASP DCE-MRI method can be used to acquire adequate high-temporal and high-spatial resolution images of the breast for contrast kinetic analysis. Despite mild compression for the MRI-guided biopsy scan, the plasma flow was different between malignant lesions and benign ones. However, the low flow level in overall may have compromised the sensitivity in the measurement of vascular permeability. Future study is warranted to include a larger cohort and to apply the method in breast MRI scans without compression.Acknowledgements
NIH R01 CA160620References
1. Saslow D, Boetes C, Burke W, Harms S, Leach MO, Lehman CD, Morris E, Pisano E, Schnall M, Sener S, Smith RA, Warner E, Yaffe M, Andrews KS, Russell CA, American Cancer Society Breast Cancer Advisory G. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA: a cancer journal for clinicians. 2007;57(2):75-89. Epub 2007/03/30. doi: 57/2/75 [pii]. PubMed PMID: 17392385.
2. Warner E, Messersmith H, Causer P, Eisen A, Shumak R, Plewes D. Systematic review: using magnetic resonance imaging to screen women at high risk for breast cancer. Ann Intern Med. 2008;148(9):671-9. Epub 2008/05/07. doi: 148/9/671 [pii]. PubMed PMID: 18458280.
3. Huang W, Tudorica LA, Li X, Thakur SB, Chen Y, Morris EA, Tagge IJ, Korenblit ME, Rooney WD, Koutcher JA, Springer CS, Jr. Discrimination of benign and malignant breast lesions by using shutter-speed dynamic contrast-enhanced MR imaging. Radiology. 2011;261(2):394-403. doi: 10.1148/radiol.11102413. PubMed PMID: 21828189; PubMed Central PMCID: PMC3198224.
4. Furman-Haran E, Schechtman E, Kelcz F, Kirshenbaum K, Degani H. Magnetic resonance imaging reveals functional diversity of the vasculature in benign and malignant breast lesions. Cancer. 2005;104(4):708-18. doi: 10.1002/cncr.21225. PubMed PMID: 15971199.
5. Heller SL, Moy L. Imaging features and management of high-risk lesions on contrast-enhanced dynamic breast MRI. AJR Am J Roentgenol. 2012;198(2):249-55. Epub 2012/01/24. doi: 198/2/249 [pii] 10.2214/AJR.11.7610. PubMed PMID: 22268165.
6. Mahoney MC, Gatsonis C, Hanna L, DeMartini WB, Lehman C. Positive predictive value of BI-RADS MR imaging. Radiology. 2012;264(1):51-8. Epub 2012/05/17. doi: 10.1148/radiol.12110619. PubMed PMID: 22589320; PubMed Central PMCID: PMC3380411.
7. Kuhl CK, Klaschik S, Mielcarek P, Gieseke J, Wardelmann E, Schild HH. Do T2-weighted pulse sequences help with the differential diagnosis of enhancing lesions in dynamic breast MRI? J Magn Reson Imaging. 1999;9(2):187-96. Epub 1999/03/17. PubMed PMID: 10077012.
8. Kim SG, Feng L, Grimm R, Freed M, Block KT, Sodickson DK, Moy L, Otazo R. Influence of temporal regularization and radial undersampling factor on compressed sensing reconstruction in dynamic contrast enhanced MRI of the breast. J Magn Reson Imaging. 2016;43(1):261-9. doi: 10.1002/jmri.24961. PubMed PMID: 26032976; PubMed Central PMCID: PMC4666836.
9. Turnbull LW. Dynamic contrast-enhanced MRI in the diagnosis and management of breast cancer. NMR Biomed. 2009;22(1):28-39. Epub 2008/07/26. doi: 10.1002/nbm.1273. PubMed PMID: 18654999.
10. Feng L, Grimm R, Block KT, Chandarana H, Kim S, Xu J, Axel L, Sodickson DK, Otazo R. Golden-angle radial sparse parallel MRI: Combination of compressed sensing, parallel imaging, and golden-angle radial sampling for fast and flexible dynamic volumetric MRI. Magn Reson Med. 2014;72(3):707-17. Epub 2013/10/22. doi: 10.1002/mrm.24980. PubMed PMID: 24142845.
11. Brix G, Kiessling F, Lucht R, Darai S, Wasser K, Delorme S, Griebel J. Microcirculation and microvasculature in breast tumors: pharmacokinetic analysis of dynamic MR image series. Magn Reson Med. 2004;52(2):420-9. doi: 10.1002/mrm.20161. PubMed PMID: 15282828.
Figures