2893
Quantitative Ultra-short Time-to-Echo Contrast-Enhanced Magnetic Resonance Imaging (qUTE-CE MRI) technique use Ferumoxytol as a Positive-Contrast Agent to Delineate Nanoparticle Accumulation in Tumors1Mechanical and Industrial Engineering, Northeastern University, BOSTON, MA, United States, 2Radiology, Massachusetts General Hospital, 3Radiology, Harvard Medical School, 4Physics, Northeastern University, Boston, MA, United States, 5Nanomedicine Science & Technology Center, Northeastern University, Boston, MA, United States, 6Psychology, Northeastern University, Boston, MA, United States
Synopsis
Here we demonstrate quantitative ultra-short time-to-echo contrast-enhanced magnetic resonance imaging (qUTE-CE MRI) technique, which employs FDA-approved ferumoxytol as a positive-contrast agent to delineate nanoparticle accumulation in tumors.
PURPOSE
Here we introduce a novel application of our previously introduced imaging modality, quantitative ultra-short time-to-echo contrast-enhanced (QUTE-CE) MRI, which uses a 3D radial UTE pulse sequence with optimized parameters at 7T that renders purely T1-weighted contrast from the administered contrast agent with little to no sensitivity to tissue heterogeneity. The technique is utilized to clearly identify and quantify regions of ferumoxytol accumulation in PC3 and FKO1 prostate tumors implanted subcutaneously in immunocompromised mice. We compare this technique to standard T1- or T2-weighted subtraction imaging and demonstrate that qUTE-CE MRI contrast is superior to those modalities.METHODS
When tumors reached about 1cm3, mice underwent three imaging sessions: pre-contrast, 1hr post-contrast, and 24hr post-contrast. Animals received a one-time i.v. bolus of 14mg/kg ferumoxytol (for a ~200µg/ml iron concentration in the blood), by i.v. administration. T1 images were taken using a RAREVTR (Rapid-Acquisition Relaxation Enhanced Variable Time Repetition). T2 images were taken using a MSME (Multi-Slice Multi-Echo) sequence. And UTE measurements were conducted using 3D-UTE pulse sequence protocol with TE=0.013ms, TR=4ms, FA=200 as described in our previous publication [1].RESULTS
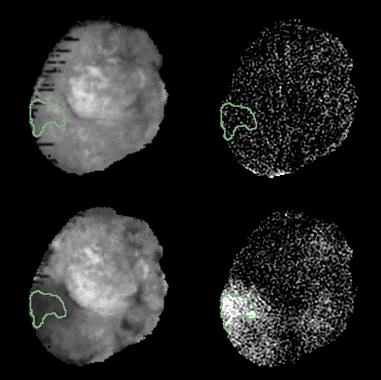
In pre-contrast images, UTE signal intensity showed a Gaussian distribution. One hour after contrast injection, UTE signal enhancement was limited to the vasculature, increasing the blood intensity by 3-5 fold. 24hr after contrast injection, ferumoxytol was cleared from the systemic circulation but residual accumulation was observed in all tumors examined. qUTE-CE MRI produced images with higher CNR than T2-weighted imaging, allowing robust quantification of ferumoxytol accumulation (Fig. 1. ). The percentage of voxels showing accumulation varied by tumor model, with PC3 tumors showing accumulation in ---% of voxels and FKO1 tumors in 1% of voxels. Ferumoxytol accumulation was experimentally manipulated by treating FKO1 tumors with radiation or nanoparticle-based Olaparib, increasing ferumoxytol accumulation by ~4-fold and ~18-fold respectively. The qUTE-CE MRI technique was sufficiently sensitive to detect these changes in accumulation.
DISCUSSION
qUTE-CE allows ferumoxytol to be used as positive contrast agent, opening up new opportunities for the use of iron oxide in MRI imaging. Being a blood pool agent, we can detect ferumoxytol in bloodstream for up to several hours after injection (half-life ~6hr in mice). Due to the enhanced permeation and retention effect of tumors, ferumoxytol can accumulate in tumors and be detected as much as 24hr after injection. Determining the exact quantity of accumulated iron oxide is difficult, since obtaining measurements outside of vasculature introduces additional complexities due to differences in relaxivity between the blood, extracellular space, and intracellular compartments. With qUTE-CE MRI it is not necessary to obtain pre-contrast images; however, here pre-contrast images proved useful for performing spatial subtractions in order to identify and quantify regions of accumulation.CONCLUSION
Here we show for the first time that qUTE-CE MRI is useful for quantifying the delivery and accumulation of nanoparticles in tumors. Given that ferumoxytol is of similar size to nanotherapeutics used in the clinic and those under development, it may prove useful as a surrogate for predicting whether nanotehrapeutics will accumulate in a given tumor.Acknowledgements
This work was supported by the following grant: NSF-DGE- 0965843References
1. Codi Gharagouzloo et al. Quantitative contrast-enhanced MRI with superparamagnetic nanoparticles using ultrashort time-to-echo pulse sequences. Magnetic Resonance in Medicine. 2015, 74(2): 431-441Figures