2885
A Comparison Study between the 2D Breath-holding and 3D Free-breathing approaches to in vivo Cardiac Quantitative Susceptibility Mapping1Meinig School of Biomedical Engineering, Cornell University, New York, NY, United States, 2Radiology, Weill Cornell Medicine, New York, NY, United States, 3Medicine, Weill Cornell Medicine, NY, United States, 4Medicine, Weill Cornell Medicine
Synopsis
Our previous study showed that high quality in vivo cardiac susceptibility maps (QSM) can be obtain using a 2D breath-hold (2DBH) sequence. However, the 2DBH approach is vulnerable to slice misregistration caused by inconsistent breath-holds, which will cause artifacts in the resulting susceptibility maps. Here we introduce a 3D navigator gated sequence (3DNAV) which allows for free-breathing during acquisition, and compare it to the 2DBH approach.
INTRODUCTION
2D breath-hold acquisition1 (2DBH) was previously used to obtain phase data for in vivo cardiac Quantitative Susceptibility Mapping (QSM). However, the 2DBH approach is very demanding on the subject as it requires the subject to have consistent breath-holds for the whole scan. This can be a very difficult task for patients with cardiovascular diseases. QSM reconstruction assumes a continuous 3 dimensional field map as the input, therefore a slice misregistration will break the continuity condition of the field map, which will result in artifacts in the final QSM image. We introduce here a 3D navigator gated sequence (3DNAV), which controls data acquisition based on diaphragm positon during free-breathing, eliminating the need for breath holds, and avoiding the problem of slice misregistration.METHODS
An ECG-triggered navigator gated multi-echo 3D FGRE sequence was developed for cardiac QSM on a 1.5T scanner (GE Healthcare). A pencil beam navigator echo was used to detect the diaphragm position, and an efficient 2-bin phase-ordered automatic window selection (PAWS) gating algorithm2 was used to control the data acquisition based on the diaphragm position in real time. Two navigator echoes were played each heartbeat, one right before acquisition and one right after acquisition. If the difference between the two diaphragm positions detected by these two navigator echoes was greater than 2mm, then the data acquired in this heartbeat are rejected. We compared the 2DBH and 3DNAV approaches for cardiac QSM in healthy volunteers.
The scan parameters for the 2DBH approach were: 1stTE/ΔTE/#TE/TR/BW = 1.5ms/2.2ms/5/15ms/±62.5kHz, acquisition resolution=192x192 with 75% phase FOV, 20 slices, 5mm slice thickness, and 10 views per heartbeat. The scan parameters for 3DNAV were the same except that 24 slices were acquired with .75NEX and .75 partial slice encoding.
Five healthy volunteers, each consented with IRB approved protocol, were scanned with the 2DBH sequence, in which they were asked to perform breath holds at maximum exhalation, and the 3DNAV sequence, in which they were freely breathing.
The total field was obtained from the complex data with a chemical shift update method3, which was initialized by the output of a graph-cut based phase unwrapping and fat-water separation method4. Then QSM was reconstructed using the Total Field Inversion Method5.
RESULTS
Three of the five volunteers were able to perform adequate consistent breath holds for successful 2DBH QSM. In comparison, 3DNAV was successfully acquired on all five volunteers, and image quality of cardiac QSM from 3DNAV was consistently better than that from 2DBH on every subject.
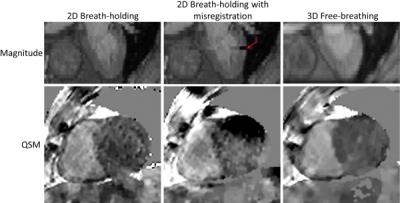
As shown in figure 1, when all breath holds were consistent, the QSM from 2DBH approach has similar RV to LV contrast as the QSM from 3DNAV approach. When there was a misregistration error caused by inconsistent breath hold (as indicated by the red arrow), then the resulting QSM had major artifacts and did not allow reliable interpretation. The QSM from 2DBH appeared to be nosier than the QSM from 3DNAV, due to lower SNR in 2DBH than in 3DNAV.
The average susceptibility difference between right and left ventricular blood pools, measured from the 2DBH QSM on the three volunteers who were able to perform adequate consistent breath holds, was 270±18ppb, which corresponded to an oxygenation level of 79.6±1.1%. The average susceptibility difference between right and left ventricular blood pools, measured from the 3DNAV QSM on the five volunteers, was 262±13ppb, which was 80±1% SvO2.
DISCUSSION
Our preliminary data showed the impracticality of the 2DBH approach for cardiac QSM. which was limited by its vulnerability to inconsistent breath holds. To understand this vulnerability, we simulated a single slice misregitration by shifting one of the slice in the volume that was successfully acquired by 2DBH approach, and we found drastically increased artifacts in QSM output. Therefore, as few as a single slice misregistration can cause major artifacts in the resulting QSM. The 3DNAV approach eliminated the need of breath holds, and the resulting cardiac QSM was uniformly better than cardiac QSM from 2DBH, making the 3DNAV approach a more viable option in clinical environment.CONCLUSION
3D acquisition with free-breathing navigator gating is a promising cardiac QSM approach without breath hold inconsistency and efforts.Acknowledgements
We acknowledge support from NIH grants R01NS072370, R01NS090464, and R01NS095562.References
1. Wen Y, Nguyen T, Liu Z, Spincemaille P, Zhou D, Dimov A, Kee Y, Kim J, Weinsaft J, Wang Y. In Vivo Quantitative Susceptibility Mapping (QSM) in Cardiac MRI. Proc Int SocMagn Reson Med 2016;24:0417.
2. Jhooti P, Gatehouse P, Keegan J, Bunce N, Taylor A, Firmin D. Phase ordering with automatic window selection (PAWS): A novel motion-resistant technique for 3D coronary imaging. MRM. 2000;43:470-480.
3. Dong J, Liu T, Chen F, Zhou D, Dimov A, Raj A, Cheng Q, Spincemaille P, Wang Y., "Simultaneous Phase Unwrapping and Removal of Chemical Shift (SPURS) Using Graph Cuts: Application in Quantitative Susceptibility Mapping," in Medical Imaging, IEEE Transactions on , 2015. 34(2), 531-540.
4. Dimov A. V., Liu T., Spincemaille P., Ecanow J. S., Tan H., Edelman R. R. and Wang Y., Joint estimation of chemical shift and quantitative susceptibility mapping (chemical QSM). Magn Reson Med, 2015, 73: 2100–2110.
5. Liu Z, Kee Y, Zhou D, Spincemaille P, Wang Y. Preconditioned total field inversion (TFI) method for quantitative susceptibility mapping. MRM. doi:10.1002/mrm.26331