2878
3D-Cine Whole-heart Magnetic Resonance Imaging Using a Novel Prospective Respiratory Self-Gating Technique1Cardiology and Pediatrics, Boston Children's Hospital and Harvard Medical School, Boston, MA, United States
Synopsis
We developed a novel prospective respiratory motion compensation algorithm, Heart-NAV, for free-breathing retrospective electrocardiogram (ECG)-gated 3D-cine steady-state free precession whole-heart magnetic resonance imaging. In 10 patients, there was good agreement between the 3D-cine and conventional breath-hold 2D-cine imaging measurements of ventricular volumes. The mean scan time for the 3D-cine acquisition was 5.9±2.7 minutes. Advantages of the Heart-NAV approach include real-time motion correction allowing for immediate in-line image reconstruction, compatibility with a variety of k-space filling approaches, and utilization of standard scanner hardware/software. Such a 3D-cine approach eliminates the need for breath-holding and simplifies planning for ventricular function assessment.
Purpose
Breath-hold, multislice 2D-cine cardiac MRI is the standard approach for assessment of ventricular function.1 This technique however, requires careful planning of multiple imaging planes by a knowledgeable operator, and repeated breath-hold instructions. In addition, young and ill patients may not be able to breath-hold leading to poor image quality. To address these deficiencies, we developed a novel prospective respiratory motion compensation technique, Heart-NAV, for high spatiotemporal resolution 3D-cine whole-heart imaging during free-breathing.Materials and Methods
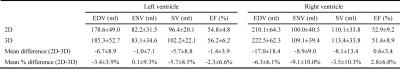
Figure 1 provides a diagram and description of the Heart-NAV technique for free-breathing retrospective ECG-gated 3D-cine steady-state free precession (SSFP) whole-heart magnetic resonance imaging. To assess the accuracy of the technique, a prospective study was performed with institutional review board approval and informed consent. Using a Philips 1.5T Achieva D-stream scanner and a 28-element phased-array coil, 10 patients with congenital heart disease (5 males; median age 33 years, range 16-50) underwent assessment of ventricular size and function with a stack of 2D-cine SSFP images in a short-axis orientation with the following parameters: FOV ≈270(SI)×270(RL)×107(AP) mm, spatial resolution 1.8×1.8×8 mm; slice gap 1 mm, heart phases 20 interpolated to 30, flip angle 60°, TE/TR 1.4/2.8 ms, bandwidth ≈1.1 kHz, and SENSE 2. The images were obtained with multiple breath-holds at end-expiration. Five to fifteen minutes after the administration of 0.15 mmol/kg gadobutral contrast, the free-breathing 3D-cine SSFP sequence with Heart-NAV for respiratory motion compensation was acquired in a sagittal orientation with the following parameters: FOV ≈512(SI)×250(AP)×180(RL) mm, isotropic spatial resolution 2.0 mm3, heart phases 20 interpolated to 30, flip angle 60°, TE/TR 1.52/3.0 ms, bandwidth ≈1.7 kHz, respiratory acceptance window 7 mm, tracking factor 1, and SENSE 3. The 3D-cine images were reconstructed in-line by the scanner and then reformatted into a short-axis, 2, 3 and 4-chamber views utilizing commercial software (Cvi-42, Circle, Calgary, Alberta, Canada). The reformatted short-axis slices matched the 2D-cine slices in thickness and gap. A single observer delineated the right and left ventricular myocardial boundaries on the short-axis 2D-cine and reformatted 3D-cine images, and was blinded to the numeric results. Left and right ventricular end-diastolic (EDV), end-systolic (ESV), stroke volume (SV), and ejection fraction (EF) were calculated from the 2D and 3D images using a standard summation of disks approach. To assess agreement, the mean of the differences (2D-3D) and mean of the differences expressed as a percentage 2x(2D-3D)/(2D+3D) were calculated.Results and Discussion
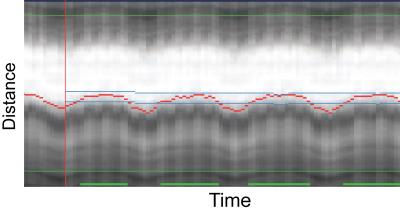
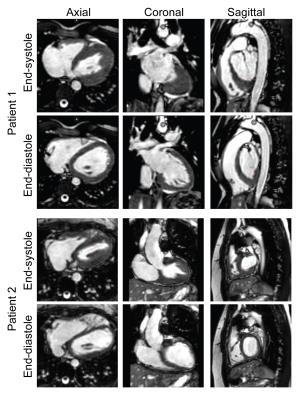
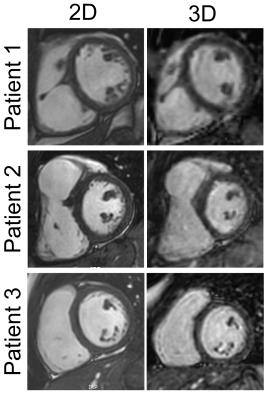
All 2D and 3D SSFP scans were successfully completed. Figure 2 shows an example of the Heart-NAV display which was used to track respiratory-induced heart motion in real-time. Figure 3 shows representative 3D-cine images in axial, coronal, and sagittal views acquired from 2 patients. Figure 4 compares mid-ventricular slices acquired in diastole from 3 patients. Mean scan time for the 3D-cine sequence was 5.9±2.7 minutes. Ventricular measurement from 2D and 3D cine data are shown and compared in Figure 5. The mean differences are in the range that is seen with inter-scan variability for patients with congenital heart disease.2 There was a systematic bias with RV and LV 3D volumes being slightly larger than 2D volumes. This discrepancy may stem from different breathing patterns (free-breathing vs. breath-hold in expiration), the lower in-plane resolution of the 3D acquisition,3 and reformatting of the 3D data. Compared to the other free-breathing 3D-cine approaches,4,5,6 the Heart-NAV technique has prospective respiratory motion correction, a higher temporal resolution, employs conventional Cartesian profile ordering, uses only ≈1.5% of cardiac cycle for motion correction, and utilizes standard hardware/software for image acquisition and reconstruction. Also, as with other studies,4,6 we used a contrast agent to enhance the blood-to-myocardium contrast-to-noise ratio. Our study is limited by a small sample size and additional patients will be studied for further validation. We utilized a navigator acceptance window of 7 mm in all subjects for consistency; future studies will explore automatic adjustment of the window during the scan to achieve a desired efficiency.Conclusion
We developed a novel prospective respiratory motion compensation algorithm, Heart-NAV, for free-breathing, retrospective ECG-gated 3D-cine SSFP whole-heart magnetic resonance imaging. Ventricular volume measurements using this technique were comparable to those obtained with a standard breath-hold 2D-cine approach, and the scan time was in a clinically acceptable range. Such a 3D-cine approach eliminates the need for breath-holding and simplifies planning for ventricular assessment.Acknowledgements
No acknowledgement found.References
1. Fratz S, Chung T, Greil GF, Samyn MM, Taylor AM, Valsangiacomo Buechel ER, Yoo SJ, Powell AJ. Guidelines and protocols for cardiovascular magnetic resonance in children and adults with congenital heart disease: SCMR expert consensus group on congenital heart disease. J Cardiovasc Magn Reson 2013;15(1):51.
2. Blalock SE, Banka P, Geva T, Powell AJ, Zhou J, Prakash A. Interstudy variability in cardiac magnetic resonance imaging measurements of ventricular volume, mass, and ejection fraction in repaired tetralogy of Fallot: a prospective observational study. J Magn Reson Imaging 2013;38(4):829-835.
3. Miller S, Simonetti OP, Carr J, Kramer U, Finn JP. MR Imaging of the heart with cine true fast imaging with steady-state precession: influence of spatial and temporal resolutions on left ventricular functional parameters. Radiology 2002;223(1):263-269.
4. Pang J, Sharif B, Fan Z, Bi X, Arsanjani R, Berman DS, Li D. ECG and navigator-free four-dimensional whole-heart coronary MRA for simultaneous visualization of cardiac anatomy and function. Magn Reson Med 2014;72(5):1208-1217.
5. Coppo S, Piccini D, Bonanno G, Chaptinel J, Vincenti G, Feliciano H, van Heeswijk RB, Schwitter J, Stuber M. Free-running 4D whole-heart self-navigated golden angle MRI: Initial results. Magn Reson Med 2015;74(5):1306-1316.
6. Han F, Zhou Z, Han E, Gao Y, Nguyen KL, Finn JP, Hu P. Self-gated 4D multiphase, steady-state imaging with contrast enhancement (MUSIC) using rotating cartesian K-space (ROCK): Validation in children with congenital heart disease. Magn Reson Med 2016 [Epub ahead of print].
Figures