2874
Improved Time Efficiency and Workflow for Fully Self-Gated Non-Contrast 5D Imaging of the Heart1Department of Radiology, University Hospital (CHUV) and University of Lausanne (UNIL), Lausanne, Switzerland, 2Center for Biomedical Imaging (CIBM), Lausanne, Switzerland, 3Advanced Clinical Imaging Technology, Siemens Healthcare AG, Lausanne, Switzerland, 4Department of Radiology, Case Western Reserve University, Cleveland, OH, United States
Synopsis
Current solutions for cardiac and respiratory motion resolved whole-heart MR imaging rely on ECG signal to synchronize data acquisition, sub-optimal strategies for fat suppression which interrupts steady-state magnetization, or contrast agent for anatomical differentiation. To address these hurdles, we present a self-gated framework with bSSFP contrast and binomial spectrally selective excitation pulse which suppresses epicardial fat signal without interrupting steady-state. When compared to existing sequences, the proposed framework reduces energy deposition and acquisition time while preserving or even improving the final image quality.
Purpose
Recent developments in cardiac MRI, which combine advanced acquisition1 and reconstruction techniques,2,3 are challenging existing paradigms by enabling simultaneous functional and anatomical 3D assessment of the whole-heart in one single scan. However, current solutions rely on ECG or navigator signals for motion artifact suppression,2 on periodic interruptions of steady-state for fat signal suppression,2 or even on contrast agents for anatomical differentiation.4 This results in inefficient data collection, prolonged scanning times,1,2 high specific absorption rates (SAR), or sub-optimal workflow. To remove these hurdles, we propose a self-gated framework that combines spectrally selective radiofrequency excitation for fat suppression, bSSFP imaging for blood-muscle contrast, and center of k-space signal extraction to remove the need for ECG and navigators. We posit that this will lead to shortened scanning times, an improved workflow, and reduced SAR when compared to earlier reports.1,2Methods
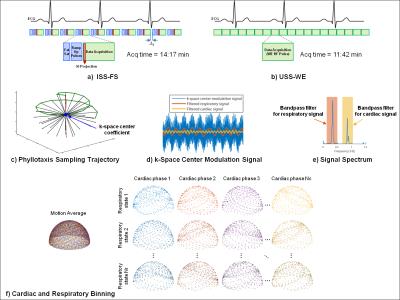
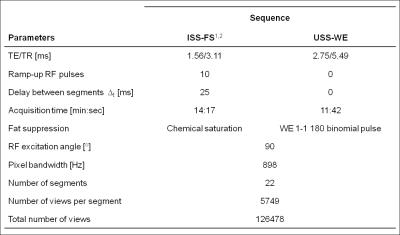
Data Acquisition: A prototype non-ECG-triggered 3D golden angle radial bSSFP sequence was used to acquire data in 3 healthy volunteers on a 1.5T clinical MRI scanner (MAGNETOM Aera, Siemens Healthcare). To preserve the steady-state magnetization, the sequence combined a segmented 3D radial trajectory5 (Fig.1c) with a 1-(180°)-1 binomial water excitation (WE) radiofrequency (RF) pulse for fat suppression (Fig.1b). The results of this uninterrupted steady-state (USS) sequence were compared to those obtained with the previously reported sequence.1 For the latter, steady-state was interrupted periodically by inserting a chemically selective preparation pulse for fat saturation (FS) and by 10 linearly increasing startup RF pulses (Fig.1a). The MR parameters of this interrupted steady-state (ISS) sequence with FS (ISS-FS) and the USS sequence with WE (USS-WE) are shown in Fig.2.
Data Sorting and Reconstruction: Self-gated cardiac and respiratory signals were extracted from the modulations of the k-space center (Fig.1d-e) and used to sort the readouts into 4 different respiratory states and 17-26 cardiac phases of 50ms window-width each. The resulting 5D (x-y-z-cardiac-respiratory dimensions) datasets were reconstructed using a k-t sparse SENSE algorithm3 that exploits sparsity along both cardiac and respiratory dimensions.
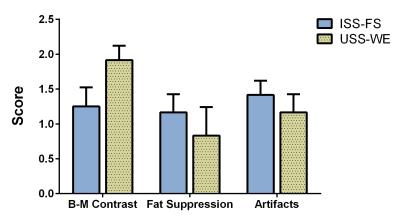
Data Analysis: The scan time and SAR associated with each sequence was recorded and compared. The 5D datasets were scored for the level of fat suppression, blood-to-myocardium contrast, and image artifacts (banding and streaking artifacts) on a scale from 0 (poor) to 2 (excellent) by two experienced readers. Reformatted images of the coronary arteries were extracted from an end-expiratory mid-diastolic position of the 5D datasets. The conspicuity and vessel length of the coronary arteries were visually compared.
Results
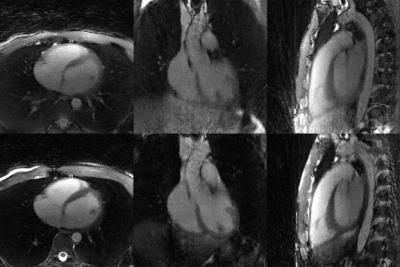
The USS-WE sequence resulted in shorter scan time (11:42min vs 14:17min) and lower SAR (56% vs 97%) than the ISS-FS sequence. No navigator or ECG signals were used for data binning. The USS-WE sequence also resulted in superior apparent blood-to-myocardium contrast compared to ISS-FS, but less effective fat suppression and more overall artifacts (Fig.3). For both techniques, cardiac and respiratory motion-resolved reconstruction effectively suppressed motion artifacts and enabled free-breathing 5D imaging of the heart. Examples of motion-resolved reconstructions from a volunteer are shown in Fig.4. Example reformats from two volunteers are displayed in Fig.5, in which the coronary conspicuity, visible vessel length and blood-muscle contrast was higher for the USS-WE sequence.Discussion
As anticipated, the proposed USS-WE sequence leads to improved time efficiency and considerably lower SAR when compared to ISS-FS. A high blood-muscle contrast was observed and suggests that contrast agents may not be needed. Together with the fact that USS-WE operates without ECG or conventional navigator signals, work flow, ease-of-use, and patient comfort clearly benefit from this proposed approach. The reduction in SAR provides the opportunity to further improve image contrast. While the WE RF pulse was reasonably effective in suppressing epicardial fat, it failed in its current form to completely suppress the fat signal from the entire chest. This may hinder the detection of motion signals and adversely affect motion-resolved reconstruction. Therefore, further refinements of the WE RF pulse as part of a bSSFP imaging sequence are warranted. Images obtained with the USS-WE sequence had fewer streaking artifacts, yet banding artifacts closer to the heart were more prominent. This can be explained by the longer TR associated with binomial RF pulsesConclusion
The proposed methodology improves time efficiency, reduces scanning time, and obviates the need for contrast agents as compared to previously reported 5D cardiac imaging approaches. An improved workflow and ease-of-use are supported by “total” self-gating where ECG or diaphragmatic navigator signals are no longer needed. While image quality was preserved, the reduction in SAR provides new opportunities for RF pulse design aimed at further improving the contrast between blood, myocardium, and fat.Acknowledgements
The authors would like to acknowledge Dr. Florian Knoll from NYU School of Medicine for support with the GPU implementation of the 3D NUFFT. This work was partly supported by the Swiss National Science Foundation grants 320030_143923 and 326030_150828.References
1. Coppo S, Piccini D, Bonanno G, Chaptinel J, Vincenti G, Feliciano H, van Heeswijk RB, Schwitter J, Stuber M. Free-running 4D whole-heart self-navigated golden angle MRI: Initial results. Magn Reson Med 2015;74(5):1306-1316.
2. Feng L, Coppo S, Piccini D, Lim RP, Stuber M, Sodickson DK, Otazo R. Five-Dimensional Cardiac and Respiratory Motion-Resolved Whole-Heart MRI. Proc Int Soc Magn Reson Med 2015; 27.
3. Feng L, Axel L, Chandarana H, Block KT, Sodickson DK, Otazo R. XD-GRASP: Golden-angle radial MRI with reconstruction of extra motion-state dimensions using compressed sensing. Magn Reson Med 2016;75(2):775-788.
4. Pang J, Sharif B, Fan Z, Bi X, Arsanjani R, Berman DS, Li D. ECG and navigator-free four-dimensional whole-heart coronary MRA for simultaneous visualization of cardiac anatomy and function. Magn Reson Med 2014;72(5):1208-1217.
5. Piccini D, Littmann A, Nielles-Vallespin S, Zenge MO. Spiral phyllotaxis: the natural way to construct a 3D radial trajectory in MRI. Magn Reson Med 2011;66(4):1049-1056.
Figures