2868
Left Atrial Enlargement and Systolic Failure Measured by Cardiac MRI in Severe Isolated Mitral Regurgitation with Preserved Left Ventricular Ejection Fraction1Auburn University MRI Research Center, Auburn University, Auburn, AL, United States, 2Electrical and Computer Engineering, Auburn University, Auburn, AL, United States, 3Division of Cardiovascular Disease, University of Alabama at Birmingham, Birmingham, AL, United States, 4Birmingham Veterans Affairs Medical Center, Birmingham, AL, United States, 5MR R&D, Siemens Healthcare, Malvern, PA, United States
Synopsis
Mitral regurgitation (MR) from degeneration of the mitral valve (MV) results in a relatively low-pressure form of volume overload caused by excess volume being ejected through a secondary ejection pathway into the left atrium (LA). Optimal timing for MV repair is under debate because a spuriously normal left ventricular (LV) ejection fraction belies severe myocardial damage and that the onset of symptom has increased risk for LV dysfunction post MV repair. LA function measured by cMRI may be an important indicator for timing of MV repair before the onset of symptoms in patients with well-preserved LV systolic function.
PURPOSE
Mitral regurgitation (MR) from myxomatous degeneration of the mitral valve (MV) results in a relatively low-pressure form of volume overload caused by excess volume being ejected through a secondary ejection pathway into the left atrium (LA). We have previously shown disruption of the sarcomeric mitochondrial architecture in patients despite a preserved LV ejection fraction in patients with isolated MR1. Previous studies have demonstrated the prognostic importance of LA size in isolated MR2-4. Cardiac MRI (cMRI) is the preferred imaging modality for the LA because volumes measured with cMRI require no geometric assumptions and are more accurate and reproducible5. We hypothesize that under the pure stretch of a regurgitant mitral valve that the LA has similar ultrastructural damage as the LV in MR and that dynamic LA volumetric dysfunction measured by cMRI predates a decrease in LVEF in isolated MR.METHODS
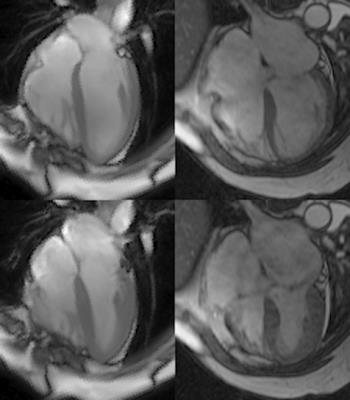
Population/Imaging protocol: MRI was acquired from 6 normal volunteers (age 19-24) and 6 patients with isolated MR (48-82) who came to mitral valve surgery for severe MR and Grade II NYHA symptoms. The normal group were imaged in a 3 Tesla (T) scanner (Siemens Healthcare, Malvern, PA), and the patients group were imaged in a 1.5T GE scanner (GE Medical Systems, Milwaukee, WI). Both groups were imaged with standard cardiac cine slices in 2 chamber and 4 chamber view, and a short-axis (SA) view covering whole ventricles and atria. Parameters were set as follows: FOV: 360-400mm, 8mm slice thickness, no gap, 256*128 matrix.
Volume Computation: In SA views, endocardial contours were manually drawn at ventricular ED and ES continuously from the LV apex to LA apex, and RV apex to RA apex. Intersections of the mitral valve (MV) and tricuspid valve (TV) leaflets with the LV and RV wall were manually placed in 2 and 4 chamber views at end diastole (ED) and end systole (ES). All intersections and endocardial contours were propagated to the remaining time frames using an automated algorithm6. Circles were fit the mitral and tricuspid annuli based on the MV and TV intersections and used to determine which contour points were part of the LV/RV and which contour points were part of the LA/RA. Chamber volumes were computed in each time frame by summing the volumes in each slice defined by the endocardial contours.
Statistics: Chamber volumes in normal volunteers and MR patients were compared with a Student’s t-test.
RESULTS
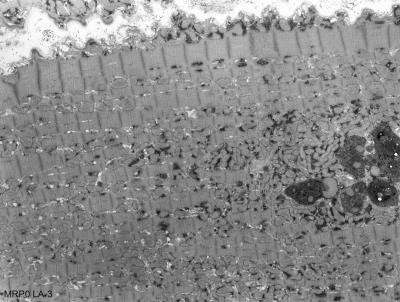
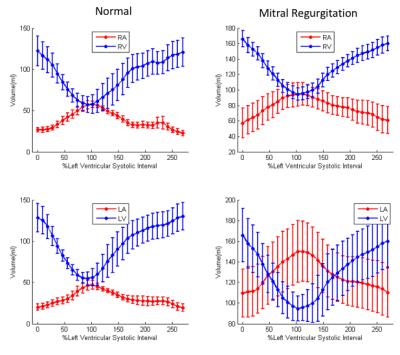
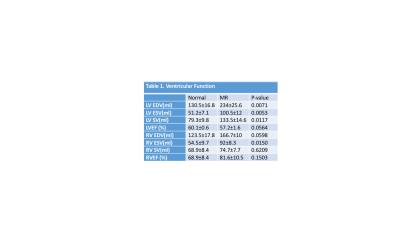
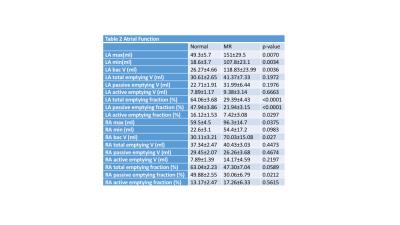
MR LVEDV was increased nearly 2-fold and LVEF did not differ from normal. In contrast, LA volume was increased 3-fold while LA emptying fraction was decreased 50% below normal. In contrast, RA and RV volume were increased by 30% with an RA emptying fraction and RVEF that did not differ from normal. Transmission electron microscopy of the LA appendage demonstrated mitochondrial disarray, blurring of z disc, myofibrillar breakdown and electron dense accumulations consistent with lipofuscin (Fig. 2). Volume time cures from each chamber (Fig. 3) demonstrate a marked 2.5 and 6-fold increase in RA and LA minimum volume respectively. It is of interest that LA minimum volume is higher than LVES; whereas RA minimum volume is 50% lower than normal RV and LV and MR RV end-systolic volume.DISCUSSION
Optimal timing for mitral valve repair is under debate because a spuriously normal LVEF belies severe myocardial damage and that the onset of symptom has increased risk for LV dysfunction post mitral valve repair. The LA contractile mechanism may more truly reflect the underlying pathology. This is because there is no inherent unloading as the LV ejects into the LA resulting in a spurious elevation of blood volume. Both LA and LV wall thickness are a match to their ejection pressures and undergo a stretch with the accompanying similar ultrastructural changes resulting from not only stretch but also neurohormonal activation. Thus, following the response of the LA may be a truer reflection of the hemodynamic and neurohormonal stress of volume overload and a better indicator for timing of surgical intervention.CONCLUSION
LA function measured by cMRI may be an important indicator for timing of mitral valve repair before the onset of symptoms in patients with well-preserved LV systolic function.Acknowledgements
No acknowledgement found.References
1. Ahmed MI, Guichard JL, Rajasekaran NS, et al. Disruption of desmin-mitochondrial architecture in patients with regurgitant mitral valves and preserved ventricular function. J Thorac Cardiovasc Surg. 2016 Oct;152(4):1059-1070
2. Le Tourneau T, Messika-Zeitoun D, Russo A, et al. Impact of left atrial volume on clinical outcome in organic mitral regurgitation. J Am Coll Cardiol. 2010 Aug 10;56(7):570-8.
3. Rusinaru D, Tribouilloy C, Grigioni F, et al. Mitral Regurgitation International DAtabase (MIDA) Investigators.. Left atrial size is a potent predictor of mortality in mitral regurgitation due to flail leaflets: results from a large international multicenter study. Circ Cardiovasc Imaging. 2011 Sep;4(5):473-81.
4. Ren B, de Groot-de Laat LE, Geleijnse ML. Left atrial function in patients with mitral valve regurgitation. Am J Physiol Heart Circ Physiol. 2014 Nov 15;307(10):H1430-7.
5. Schiros CG, Dell'Italia LJ, Gladden JD, et al. Magnetic resonance imaging with 3-dimensional analysis of left ventricular remodeling in isolated mitral regurgitation: implications beyond dimensions. Circulation. 2012 May 15;125(19):2334-42.
6. Feng W, Nagaraj H, Gupta H, et al. A dual propagation contours technique for semi-automated assessment of systolic and diastolic cardiac function by CMR. J Cardiovasc Magn Reson. 2009 Aug 13;11:30.
Figures