2851
Image-based Background Phase Error Correction in 4D Flow MRI1Institute for Biomedical Engineering, University and ETH Zurich, Zurich, Switzerland, 2Department of Radiology, University Hospital Cologne, 3Division of Imaging Science and Biomedical Engineering, King’s College London
Synopsis
Background phase errors occurring in 4D Flow MRI are analyzed with respect to their spatial order. Results demonstrate that background errors can range from first up to third spatial order requiring correction with appropriate polynomial orders. While higher order corrections perform well even for low SNR, they are highly sensitive to the amount of stationary tissue present for background phase estimation requiring at least 25%, 60% and 75% of stationary tissue for systems with first, second and third order offsets The amount of stationary tissue available in-vivo, however, limits the use of higher order polynomial models for background phase correction.
Purpose
4D Flow MRI is of increasing interest and use for various applications1,2. To correct for residual background phase errors, image-based correction approaches by referencing through stationary tissue are widely used in practice3-5. It is the aim of the present study to analyze background phase errors occurring in 4D Flow MRI.Material and Methods
4D Flow MRI data was acquired for typical geometries and encoding velocities in a stationary phantom on two clinical Philips MR systems. Sequence parameters were chosen according to recommendations of the 4D Flow MRI consensus statement6. Background errors were analyzed with respect to their spatial order. Minimum requirements regarding the signal-to-noise ratio (SNR) and the amount of stationary tissue necessary for phase error estimation were assessed. On the phantom data, limited amount of stationary tissue for linear regression was simulated by assigning an elliptical volume of varying size in the center of the phantom data as non-stationary, thereby leaving the rim of the phantom as "stationary tissue".
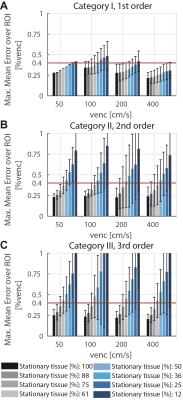
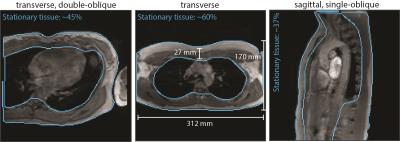
For comparison, volumes and locations of stationary tissue were derived from in-vivo data. Additionally for in-vivo validation, 4D Flow data of the aorta was acquired in 5 healthy subjects on one MR system including subsequent scans on the stationary phantom.
To assess the quality of linear regression the root-mean-square error (RMSE) was used. The RMSE is stated in % of the encoding velocity (%venc) and was calculated over the full imaged object volume. In addition, for the phantom study the mean error over all possible cubic regions-of-interest (ROIs) of 30x30x30mm3 was calculated and the maximum extracted: $$\max M{{E}_{ROI}}=\underset{ROIs}{\mathop{\max}}\,\left(\left|\frac{\sum\nolimits_{i\in ROI}{\left({{v}_{i}}-{{{\tilde{v}}}_{i}}\right)}}{{{N}_{ROI}}} \right|\right)$$
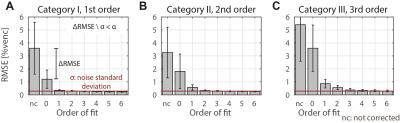
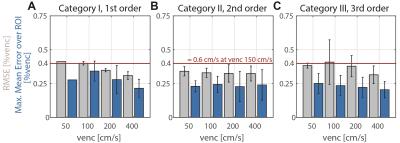
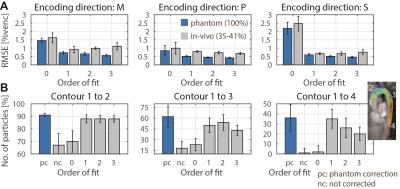
where $$$v_{i}$$$ is the measured data and $$${{\tilde{v}}_{i}}$$$ the estimated background phase at voxel $$$i$$$. The edge length of the ROIs was chosen to be 30mm in accordance to a typical aortic diameter. The maxMEROI was evaluated over the inner 50% of the imaged object volume excluding regions which would be stationary in-vivo. A threshold of the maxMEROI of 0.4 %venc was defined which would translate into an error of 0.6 cm/s for an encoding velocity of 150 cm/s which in turn corresponds to 5% error in stroke volume as a limit of acceptability7 for clinical applications. For in-vivo validation, particle trace counts at three locations in the aorta were computed. To analyze the spatial variation of the background error, the relative improvement in RMSE between consecutive spatial orders was analyzed and a threshold was set at 30% of the noise level defined as the RMSE after a sixth order correction.
Results
On the two MR systems used and for the different settings, spatial variation of background errors up to third spatial order were measured (Figure 1) and data was grouped into according categories, i.e. data in category I shows spatial variation of the background error up to first order. The maxMEROI was below 0.4 %venc for all encoding velocities upon first, second and third order correction for category I, II and III, respectively (Figure 2). The residual error did not change significantly down to an SNR of 15 independently of the spatial order. The minimum amount of stationary tissue, however, was strongly dependent on the spatial order requiring at least 25%, 60% and 75% of stationary tissue for systems with first, second and third order offsets (Figure 3). In the volunteers measured, however, the amount of stationary tissue ranged from 35 to 63% of the total object volume depending on the geometry with an SNR ranging from 20 to 50 (Figure 4). The in-vivo validation of the aorta showed that with approximately 35-40% of stationary tissue available only first order correction yields a significant reduction in background phase error as exemplified by increased particle trace counts (Figure 5). Nonetheless, first order correction resulted in increased errors and reduced particle tracking accuracy when compared to the phantom correction using a third order polynomial model for estimation of the background offsets (Figure 5).Discussion
Background phase errors can range from first to third spatial order requiring correction with appropriate polynomial orders. It is therefore recommended to test and adapt the order of background phase error correction for each individual MR system type and, accordingly, implement lower limits of the SNR and amount of stationary tissue depending on the polynomial order required for background phase correction. At the same time, however, the limited amount of stationary tissue typically available in-vivo limits image-based background phase error correction to zeroths and first spatial order despite the presence of higher order background phase errors. To this end, alternative correction methods including magnetic field monitoring8-10 need to be studied further.Acknowledgements
The authors thank Martin Bührer for support with the MRecon software package and acknowledge funding from the Swiss National Science Foundation, grant 320030_149567.References
1. Markl M, Frydrychowicz A, Kozerke S, Hope M, Wieben O: 4D flow MRI. J Magn Reson Imaging 2012, 36:1015–36.
2. Srichai MB, Lim RP, Wong S, Lee VS: Cardiovascular Applications of Phase-Contrast MRI. Am J Roentgenol 2009, 192:662–675.
3. Caprihan A, Altobelli S, Benitez-Read E: Flow-velocity imaging from linear regression of phase images with techniques for reducing eddy-current effects. J Magn Reson 1990, 90:71–89.
4. Walker PG, Cranney GB, Scheidegger MB, Waseleski G, Pohost GM, Yoganathan AP: Semiautomated method for noise reduction and background phase error correction in MR phase velocity data. J Magn Reson Imaging 1993, 3:521–30.
5. Lankhaar J-W, Hofman MBM, Marcus JT, Zwanenburg JJM, Faes TJC, Vonk-Noordegraaf A: Correction of phase offset errors in main pulmonary artery flow quantification. J Magn Reson Imaging 2005, 22:73–9.
6. Dyverfeldt P, Bissell M, Barker AJ, Bolger AF, Carlhäll C-J, Ebbers T, Francios CJ, Frydrychowicz A, Geiger J, Giese D, Hope MD, Kilner PJ, Kozerke S, Myerson S, Neubauer S, Wieben O, Markl M: 4D flow cardiovascular magnetic resonance consensus statement. J Cardiovasc Magn Reson 2015, 17:72.
7. Gatehouse PD, Rolf MP, Bloch KM, Graves MJ, Kilner PJ, Firmin DN, Hofman MBM: A multi-center inter-manufacturer study of the temporal stability of phase-contrast velocity mapping background offset errors. J Cardiovasc Magn Reson 2012, 14:72.
8. Giese D, Haeberlin M, Barmet C, Pruessmann KP, Schaeffter T, Kozerke S: Analysis and correction of background velocity offsets in phase-contrast flow measurements using magnetic field monitoring. Magn Reson Med 2012, 67:1294–302.
9. Busch J, Vannesjo SJ, Barmet C, Pruessmann KP, Kozerke S: Analysis of temperature dependence of background phase errors in phase-contrast cardiovascular magnetic resonance. J Cardiovasc Magn Reson 2014, 16.
10. Vannesjo SJ, Graedel NN, Kasper L, Gross S, Busch J, Haeberlin M, Barmet C, Pruessmann KP: Image reconstruction using a gradient impulse response model for trajectory prediction. Magn Reson Med. 2016, 76(1):45-58
Figures