2834
Evaluation of Aortic Hemodynamics in Patients with Sievers Type II BAV1Radiology, Northwestern University, Chicago, IL, United States, 2Radiology, Lurie Children's Hospital, Chicago, IL, United States, 3Biomedical Engineering, Northwestern University, Chicago, IL, United States
Synopsis
Sievers type II bicuspid aortic valve (BAV) is a rare disease that has been associated with more severe aortopathy than the more common type I BAV. To study the relation between aortic valve (AV) morphology and altered aortic hemodynamics in this disease, 4D flow MRI data from 32 type II BAV patients with different AV fusion types were analyzed. The helicity direction of blood flow in the ascending aorta was found to be influenced by AV morphology. Additional research with a larger cohort and comparison to type I BAV should render further insights into the pathophysiologic mechanism underlying BAV aortopathy.
Introduction
With an estimated incidence of 0.02%, Sievers type II bicuspid aortic valve (BAV) is considered a rare subtype of BAV disease [1]. It is associated with more severe aortopathy than the more frequently found type I BAV, including aortic valve stenosis and regurgitation as well as progressive aortic dilatation and dissection [2, 3]. Recent 4D flow MRI studies provide evidence that altered aortic 3D blood flow patterns in BAV are associated with the development of aortopathy and aortic wall degeneration [4, 5]. However, few studies have investigated aortic hemodynamics in patients with type II BAV as a potential pathophysiologic mechanism of disease development [6]. Three-dimensional evaluation of complex flow patterns seen in type II BAV could provide new insights into the relation between valve morphology, altered aortic hemodynamics and aortopathy in these patients, and eventually improve risk stratification. This study aimed to apply aortic 4D flow MRI to study changes in aortic blood flow in type II BAV patients with different valve morphologies.Methods
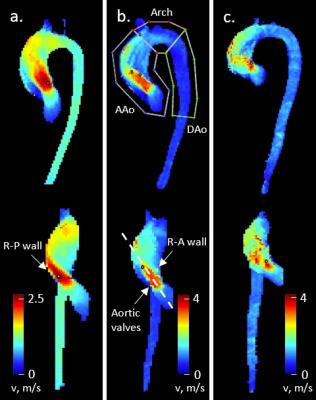
A retrospective query identified 32 patients with right-left/right-non (RL/RN) aortic valve fusion who underwent cardiothoracic MRI including 4D flow MRI for surveillance of the thoracic aorta. Demographics are listed in Table 1. Aortic valve morphology was assessed using 2D CINE SSFP and 2D phase-contrast MRI images obtained at 1.5T. 4D flow data were acquired during free breathing using diaphragm navigator gating with spatial resolution = 2.8-3.4 x 2.1-2.4 x 2.5-3.5 mm, temporal resolution = 36.8-39.2 ms and 3-directional velocity encoding with VENC = 150-450 cm/s. 4D flow MRI data analysis included pre-processing (noise masking, phase offset error corrections, velocity anti-aliasing), calculation of a 3D PC-MR angiogram, and 3D segmentation of the aorta. Time-resolved 3D pathlines (Ensight, CEI, USA) were used to grade flow helicity in the ascending aorta by two independent observers (grade 0: no helicity, grade 1: flow rotation of <360°, grade 2: flow rotation of >360°, grade 3: flow rotation of >720°). Maximum intensity projections (MIPs) showing maximum velocities at peak systole were used to compare systolic flow jet orientations and to quantify peak systolic flow velocities in the ascending aorta, aortic arch and descending aorta (Figure 1, top). For statistical analysis of differences between groups, Kruskal-Wallis H tests and Mann-Whitney U tests were performed with a p-value of 0.05. Categorical data were evaluated with Pearson’s chi square test. Inter-observer variability was computed using the kappa statistic.Results
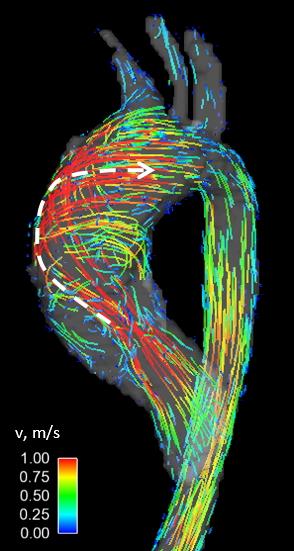
The cohort was divided into four groups with different presentations of partial and entire RL- and RN-fusion (Table 1). Good interobserver agreement was obtained for both helicity grading (κ = 0.69) and assessment of systolic flow jet orientation (κ = 0.73). Systolic flow jets were either directed toward the right-posterior wall of the ascending aorta or along the vessel’s long axis, or were not identifiable (Figure 1). An overview of observed hemodynamics is given in Table 2. No differences between morphology groups were found in helicity grades, peak systolic velocities and flow jet orientations, as well as SOV and MAA diameters. Helicity direction was more frequently right-handed for eRL/pRN-fusion than for eRL/eRN-fusion (p = 0.009) and eRN/pRL-fusion (p = 0.008) and more frequently left-handed for pRL/pRN-fusion than for eRL/pRN-fusion (p < 0.001). Figure 2 shows an example of a right-handed helical flow pattern for a patient with eRL/pRN-fusion. Patients with pRL/pRN-fusion were significantly younger than patients with eRL/eRN-fusion (p = 0.003) and pRN/eRL-fusion (p = 0.008).Discussion
The most pronounced helical blood flow patterns in type II BAV patients were seen for eRL/pRN- and pRL/pRN-fusion of the aortic valves, although the assigned helicity grades were not significantly different. However, the distinct helicity directions in these two groups (right-handed and left-handed, respectively) demonstrate that aortic hemodynamics are influenced by small variations in valve morphology. The markedly lower age seen for pRL/pRN-fusion might indicate a faster disease development in this group of patients. A larger cohort and additional evaluation of aortic valve regurgitation as a confounder is needed to further investigate these patterns. This study is the first to evaluate aortic hemodynamics in a group of type II BAV patients with different aortic valve morphologies. Future studies should include comparisons with type I BAV and control groups of healthy TAV patients to investigate whether the more severe aortopathy seen in type II BAV finds its cause in more aberrant aortic flow patterns.Conclusion
Varying aortic valve morphologies in type II BAV disease induce subtle differences in aortic hemodynamics. Additional research can render further insights into the pathophysiologic mechanisms of BAV disease and thereby facilitate improved risk stratification.Acknowledgements
Grant funding by NIH R01 HL115828 and K25 HL119608.References
1. Novaro, G.M., M. Mishra, and B.P. Griffin, Incidence and echocardiographic features of congenital unicuspid aortic valve in an adult population. J Heart Valve Dis, 2003. 12(6): p. 674-8.
2. Mookadam, F., et al., Unicuspid aortic valve in adults: a systematic review. J Heart Valve Dis, 2010. 19(1): p. 79-85.
3. Sievers, H.H., et al., New insights in the association between bicuspid aortic valve phenotype, aortic configuration and valve haemodynamics. Eur J Cardiothorac Surg, 2016. 49(2): p. 439-46.
4. Mahadevia, R., et al., Bicuspid aortic cusp fusion morphology alters aortic three-dimensional outflow patterns, wall shear stress, and expression of aortopathy. Circulation, 2014. 129(6): p. 673-82.
5. Guzzardi, D.G., et al., Valve-Related Hemodynamics Mediate Human Bicuspid Aortopathy: Insights From Wall Shear Stress Mapping. J Am Coll Cardiol, 2015. 66(8): p. 892-900.
6. Entezari, P., et al., From unicuspid to quadricuspid: influence of aortic valve morphology on aortic three-dimensional hemodynamics. J Magn Reson Imaging, 2014. 40(6): p. 1342-6.
Figures