2823
Cardiac manifestations of diffuse lung disease: A pictorial review of cardiac magnetic resonance imaging findings1Department of Radiology, University of Wisconsin-Madison, Madison, WI, United States, 2Department of Medical Physics, University of Wisconsin-Madison, Madison, WI, United States, 3Department of Pediatrics, University of Wisconsin-Madison, Madison, WI, United States
Synopsis
Many disease processes associated with diffuse lung disease also have cardiac abnormalities. These cardiac abnormalities can easily be overlooked if one focuses too heavily on the lung findings. The purpose of this educational exhibit is to review the characteristic imaging findings associated with the cardiac manifestations of diffuse lung disease.
Purpose:
Extensive parenchymal changes of diffuse lung disease may distract from associated cardiac involvement due to the satisfaction of search for the imaging physician. Conduction problems, constrictive pericarditis, myocarditis and pulmonary arterial hypertension are often found in diffuse lung diseases (DLD). We will review imaging findings of the cardiac manifestations of those DLD that may also affect the heart.Content Organization:
The following pathological conditions will be presented in a pictorial essay format:
Connective Tissue Diseases:
Systemic lupus erythematosus (SLE) - Valvular thickening and vegetations, most commonly along the mitral and aortic valves [1], can be seen in SLE along with pericardial effusions and thickening of the pericardium. Patients may also present with acute myocarditis.
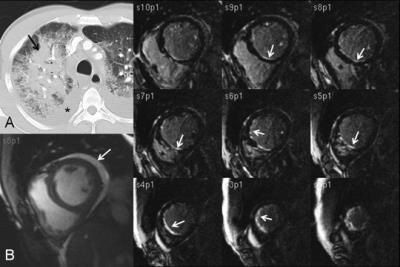
Systemic sclerosis (SS- Scleroderma) - Myocardial fibrosis can occur with delayed myocardial enhancement seen in approximately 20% of patients. Patients may also have right and left ventricular dysfunction[2]. (Fig. 1) These patients will have non-specific interstitial fibrosis (NSIP) and esophageal dilation as well.
Polymyositis - Histopathological changes of myocarditis may be present along with abnormal delayed myocardial enhancement in a pattern similar to infectious myocarditis.
Infection:
Tuberculosis - Diffuse pericardial thickening and pericardial effusion can lead to cardiac tamponade.
Viral pneumonia and myocarditis - Patchy delayed myocardial enhancement can be seen in patients with viral myocarditis[3]. (Fig. 2)
Vasculitis:
Churg-Strauss - Cardiac disease is common in up to 60% patients and is the leading cause of death. Abnormal delayed myocardial enhancement is seen in the majority of patients.
Behcet’s - Small vessel vasculitis can lead to coronary artery aneurysms and thrombosis. Mitral valve prolapse and regurgitation may also be present along with right atrial and ventricular thrombosis. This disease can also be associated with pulmonary artery aneurysms.
Drug toxicity: The cardiac findings are diverse and depend on the type of drug involved.
Cocaine – A common cause of myocardial ischemia and infarct in those individuals less than 30 years of age.
Anthracyclines: (Adriamycin/ Doxorubicin): Mitochondrial toxicity with resultant cardiomyopathy which is commonly seen in breast cancer survivors.
Sarcoid reactions: This is an unusual reaction to prescription medication that typically only affects the lungs and spares the myocardium.
Radiation therapy: Localized therapy to the chest or whole body radiation can cause a variety of cardiac pathologies including pericarditis, thickening and calcification of the mitral and aortic valves, coronary artery stenosis and left ventricular diastolic and systolic dysfunction. This is most commonly seen in survivors of Hodgkin’s Disease.
Neoplastic/metastatic: Metastatic lesion from the breast and lung cancers are the most common cardiac masses.
Infiltrative:
Hypereosinophilia syndrome (HES): Loeffler's endocarditis occurs in the majority of patients with HES. Acute eosinophilic infiltration of endocardium and myocardium results in thrombosis, inflammation, and fibrosis of intramural coronary arteries. Mural thrombosis and endocardial fibrosis is also common. (Fig. 3)
Sarcoidosis: Cardiac involvement is seen in up to 40% of patients at autopsy. Non-caseating granulomas in the myocardium lead to mid myocardial and subepicardial delayed myocardial enhancement [4,5]. (Fig. 4) Of all the chest diseases, this is the most common that the imager will deal with in practice.
Amyloidosis: Cardiac involvement is a frequent cause of death in patients with amyloid. Over 90% of patients will have abnormal delayed gadolinium enhancement on MRI most commonly in a diffuse pattern. It is often difficult to properly null the myocardium.
Summary:
Cardiac involvement in patients with diffuse lung disease is an important cause of morbidity and mortality in these patients that can be easily overlooked. Diffuse lung diseases have distinct patterns of cardiac involvement that can be easily characterized with cardiac CT and MRI, but will remain occult on CXR.Acknowledgements
No acknowledgement found.References
1. Roldan C, et.al. NEJM 1996;335(19):1424-30.
2. Nassenstein K, et al. Rofo. 2008; 180:1054-1060.
3. Pisani B, et al. Am J Med 1997;102:459.
4. Mehta D, et al. Chest 2008;133:1426-35.
5. Smedema J, et al. Chest 2005;128:1629-37.
Figures
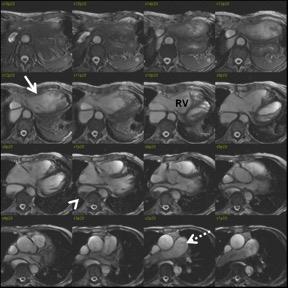
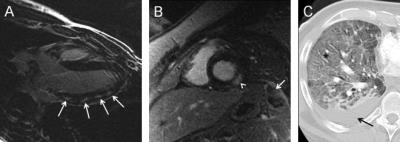
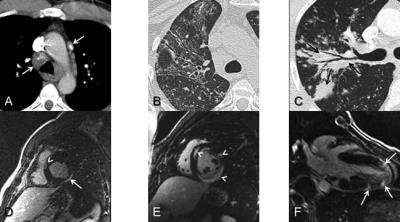
Figure 2. (A) Patchy mid-myocardial enhancement (arrows) in lateral wall of left ventricle in patient with acute viral myocarditis.
(B) Ebstein-Barr Virus (EBV) myocarditis in patient with mononucleosis. Myocardial delayed enhancement image shows focal subepicardial enhancement in the inferolateral wall (arrowhead) and splenic infarct (arrow).
(C) Acute viral pneumonia (HSV and influenza A) presenting with diffuse lung consolidation and reticulation due to diffuse alveolar damage and edema (*) and a small right pleural effusion (arrow).