2822
Direct and Indirect Findings of Pulmonary Embolism Using Contrast Enhanced Magnetic Resonance Angiography (CE-MRA)1Department of Radiology, University of Wisconsin-Madison, Madison, WI, United States, 2Department of Medical Physics, University of Wisconsin-Madison, Madison, WI, United States, 3Department of Pediatrics, University of Wisconsin-Madison, Madison, WI, United States, 4Department of Medicine, University of Wisconsin-Madison, Madison, WI, United States, 5Department of Emergency Medicine, University of Wisconsin-Madison, Madison, WI, United States, 6Department of Biomedical Engineering, University of Wisconsin-Madison, Madison, WI, United States
Synopsis
Contrast enhanced pulmonary MRA is an important modality for detecting PE in those situations where there are concerns about excess exposure to ionizing radiation or contraindications to iodinated contrast. While most radiologists are experienced in interpreting CT angiographic studies for pulmonary embolus, many are unfamiliar with CE-MRA for the diagnosis of PE. It is important for interpreting physicians to understand both the direct and indirect findings associated with PE. In our retrospective study of 682 patients, we found 136 PE in 61 patients. The prevalence of both direct and indirect findings associated with PE were reviewed in this study.
PURPOSE:
The purpose of this work was to determine the prevalence of direct and indirect findings of pulmonary embolism (PE) on pulmonary MRA studies obtained for the primary diagnosis of PE.METHODS:
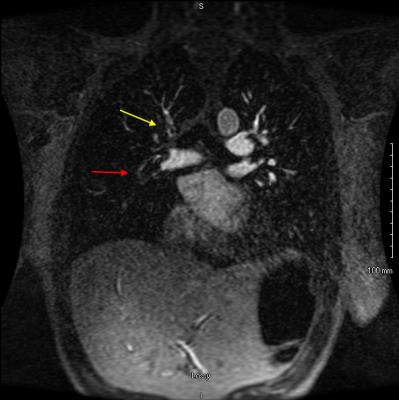
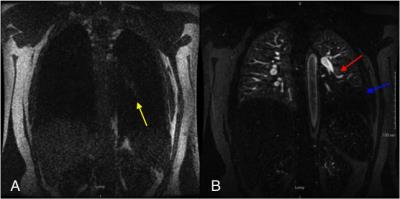
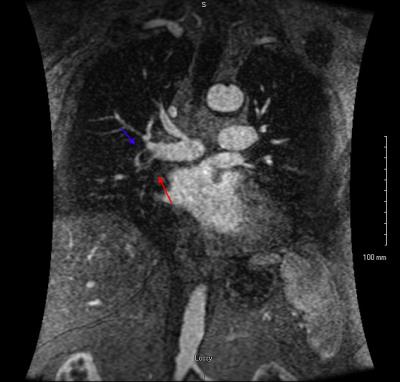
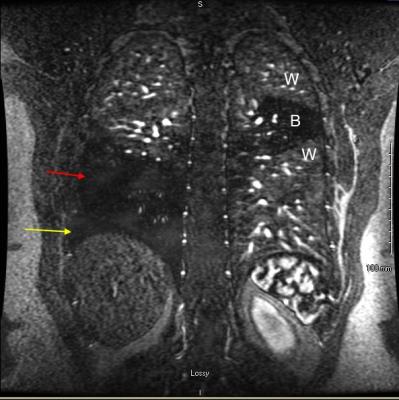
After obtaining IRB approval, a retrospective review of 682 CE-MRA exams performed for the primary diagnosis of PE was performed to assess the prevalence of various direct and indirect findings associated with PE. There were 61 patients with at least one PE identified and reviewed. Direct findings that were evaluated included: occlusive clot, non-occlusive clot (Fig. 1), clot to vessel signal intensity (S.I.) ratio, vessel cutoff, high T1 weighted signal intensity clot (relative to the non-enhanced pulmonary artery on the non-contrast T1 weighted acquisition, Fig. 2) and the double bronchus sign (Fig. 3)[3]. Indirect findings evaluated included the following: pulmonary infarction on T2 weighted sequences, perfusion defects (Fig. 2,4), pleural effusion, enhancing parietal pleura, pulmonary venous stasis, vessel wall enhancement, atelectasis and an interrupted white-black-white interface of a perfusion defect within an area of atelectasis (Fig. 4).RESULTS:
We found a total of 136 PE in the 61 patients. The prevalence of direct findings of PE was as follows: 72/136 (53%) occlusive clot, 64/136 (47%) non-occlusive clot, 0.26 ± 0.16 average (± S.D.) clot to vessel signal intensity ratio, 55/136 (40%) vessel cutoff, 19/136 (14%) high T1 weighted signal intensity clot and 56/136 (41%) cases of the double bronchus sign. The prevalence of indirect findings was as follows: 18/136 (13%) pulmonary infarcts on T2 weighted sequences, 73/136 (54%) perfusion defects, 24/136 (18%) pleural effusion, 21/136 (15%) enhancing parietal pleura, 36/136 (26%) pulmonary venous stasis, 16/136 (12%) vessel wall enhancement, 34/136 (25%) atelectasis and 16/136 (12%) instances of the white-black-white interface.DISCUSSION:
CE-MRA is becoming an increasingly important modality for detecting PE especially in those situations where there are concerns about excess exposure to ionizing radiation or contraindications to iodinated contrast[1,2]. While most radiologists are experienced in the interpretation of pulmonary CTA studies, many are uncomfortable performing and interpreting pulmonary CE-MRA. When a high quality exam is performed, large PE can easily be seen directly. When the quality of the exam is limited or the PE is small, knowledge of indirect findings can assist in localizing PE or determining its likely presence.CONCLUSION:
Both direct and indirect finding of pulmonary embolism have been described at pulmonary MRA. We have assessed the prevalence of both expected direct and indirect findings of PE some of which are also seen at computed tomographic angiography (CTA) while others are new observations that are unique to MRA.Acknowledgements
We wish to acknowledge research support to the Department of Radiology from GE Healthcare.References
1. Schiebler ML, Nagle SK, Francois CJ, et al. Effectiveness of MR angiography for the primary diagnosis of acute pulmonary embolism: clinical outcomes at 3 months and 1 year. Journal of magnetic resonance imaging : JMRI 2013;38(4):914-925.
2. Nagle SK, Schiebler ML, Repplinger MD, et al. Contrast enhanced pulmonary magnetic resonance angiography for pulmonary embolism: Building a successful program. Eur J Radiol 2016;85(3):553-563
3. Schiebler ML, Lindholm C, Francois C, Reeder S, Grist TM, Vigen K, Repplinger M, Nagle S. The Double Bronchus sign: A new observation of occlusive pulmonary embolism at pulmonary MRA. Oral.presentation MRA Club, Rome, Italy, Sept 19, 2014
Figures