2808
Non-Contrast-Enhanced MRA for Hepatic Vasculature Imaging using Off-Resonance-Robust Velocity-Selective Pulse Train1Philips Healthcare, Shanghai, People's Republic of China, 2Radiology, Johns Hopkins University, Baltimore, United States, 3F.M. Kirby Research Center for Functional Brain Imaging, Kennedy Krieger Institute, Baltimore, United States, 4Radiology, Shanghai General Hospital, Shanghai, People's Republic of China, 5Center for Biomedical Imaging Research, Department of Biomedical Engineering, School of Medicine, Tsinghua University, Beijing, People's Republic of China
Synopsis
Preoperative hepatic vascular evaluation is of great importance in a variety of liver surgeries, including tumor resection and transplantation. An accurate depiction of hepatic vascular anatomy can help to prevent the complications and decrease the morbidity and mortality in liver surgeries. However, few studies have reported good image quality in hepatic vasculature imaging using non-contrast-enhanced MR angiography (NCE-MRA) because of the motion and complex structure. In recent years, some studies reported the potential of NCE-MRA with velocity-selective pulse train (VSMRA). In this study, we demonstrated the feasibility of VSMRA for imaging the hepatic vasculature with an off-resonance-robust velocity-selective pulse train at 3T.
Purpose
Preoperative hepatic vascular evaluation is of great importance in a variety of liver surgeries, including tumor resection and transplantation. An accurate depiction of hepatic vascular anatomy can help to prevent the complications and decrease the morbidity and mortality in liver surgeries [1-2]. Non-contrast-enhanced MR angiography (NCE-MRA) has been widely used in clinical applications because it is noninvasive, free of radiation and not requiring contrast agents. However, for hepatic NCE-MRA, it is not easy to obtain images with acceptable quality due to motion and relatively complex vasculature of the liver. Recently, velocity-selective magnetization-prepared MRA (VSMRA) has shown good image quality in cerebral, renal, and peripheral regions [3-6]. In this study, we aim to image the hepatic vasculature with an off-resonance-robust velocity-selective (VS) pulse train at 3T [5].Method
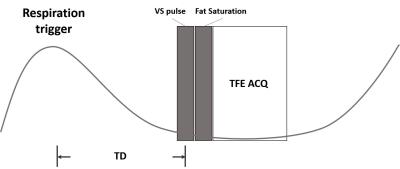
In this study, we used the off-resonance-robust VS pulse train [5], which can improve the image quality in the abdominal imaging with a large FOV. The sequence diagram for VSMRA is shown in Fig. 1. Respiration trigger is used to suppress the motion artifact. After a delay time (TD), VS pulse train is applied and then a fat suppression pulse follows. Data are acquired with a turbo field echo (TFE) sequence.
Experiments were performed on a Philips 3.0T Achieva TX or a 3.0T Ingenia MRI scanner (Philips Healthcare, Best, The Netherlands) with a phased-array 32-channel cardiac coil or a phased-array 32-channel dS Torso coil, respectively. Four healthy volunteers were employed in this study. We used two types of parameters with different spatial resolutions to evaluate the image quality. For the high resolution protocol, the scan parameters were TR/TE=9.6/5.7ms, FA=15$$$^\circ$$$, acquisition voxel size=1.0×1.0×2.0mm3, FOV=240×240×80mm3, BW=192.9Hz/pixel, TFE factor=40, cut-off velocity=5cm/s, field of speed (FOS)=45cm/s, scan time=4min6sec with SENSE factor=3. Two consecutive VS pulse trains were applied to suppress the tissue background more efficiently. For the low resolution protocol, the scan parameters were TR/TE=7.3 /4.4ms, FA=15$$$^\circ$$$, acquisition voxel size=1.2×1.2×2.4mm3, FOV=240×240×80mm3, BW=289.4Hz/pixel, TFE factor=30, scan time=3min39sec with SENSE factor=3, the VS pulses applied were the same as the ones in the high resolution protocol. As the directions of most hepatic vessels have the right-left (RL) direction component, the velocity encoding gradients in the VS pulse train were placed in the RL direction.
Result
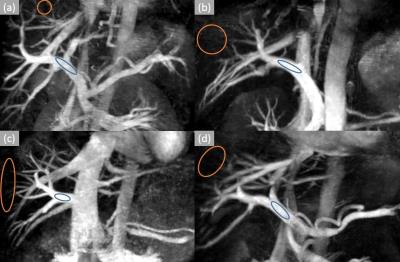
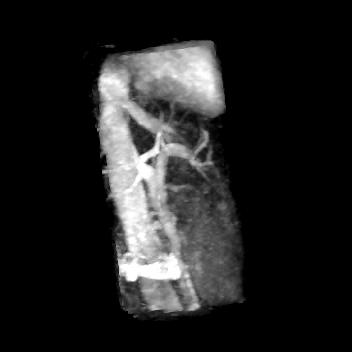
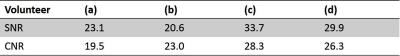
Fig. 2 (a), (b), (c), (d) show the results of hepatic VSMRA in all volunteers respectively. Maximum intensity projection (MIP) was used to present the hepatic vasculature. Among these four volunteers, high spatial resolution protocol was used for volunteers (a) and (b), low spatial resolution protocol was used for volunteers (c) and (d). Fig. 3 shows the results of volunteer (c) after MIP rotation from different view angles. The SNR and CNR of the main portal veins were listed in Table 1, and the ROIs for SNR and CNR calculation are indicated by blue and orange circles in Fig. 2.Discussion
The results show the feasibility to image the hepatic vasculature with the VSMRA technique. In VSMRA images, the hepatic portal veins and hepatic veins can be clearly identified and the third level branches can be shown in all volunteers in protocols with both high and low spatial resolution. With two consecutive VS pulse trains, the background signal can be better suppressed. In comparison with NCE-MRA using time spatial labeling inversion pulse (Time-SLIP) [7], VSMRA doesn’t rely on the flow-in effect so that we don’t need to optimize the inversion time (TI) which varies with each individual in the sequence. The independence from TI can improve the robustness of this technique among different people. In addition, the hepatic vein can be shown with the hepatic portal vein simultaneously which provides a better depiction of the hepatic vasculature.Conclusion
An NCE-MRA method using off-resonance-robust velocity-selective pulse train for hepatic vasculature imaging is proposed in this work. The experiments show its potential to serve as a robust way to provide high-quality images in hepatic vasculature imaging.Acknowledgements
No acknowledgement found.References
1. Dushyant Sahani, et al. Preoperative Hepatic Vascular Evaluation with CT and MR Angiography: Implications for Surgery. RadioGraphics, 2004; 24(5): 1367-1380.
2. William B. Eubank, et al. Preoperative Evaluation of Patients Awaiting Liver Transplantation: Comparison of Multiphasic Contrast-Enhanced 3D Magnetic Resonance to Helical Computed Tomography Examinations. JMRI, 2002; 16: 565–575.
3. Taehoon Shin, et al. Non-Contrast-Enhanced Renal and Abdominal MR Angiography Using Velocity-Selective Inversion Preparation. MRM, 2013; 69: 1268–1275.
4. Taehoon Shin, et al. Off-Resonance-Robust Velocity-Selective Magnetization Preparation for Non-Contrast-Enhanced Peripheral MR Angiography. MRM, 2013; 70: 1229–1240.
5. Qin Qin, et al. Velocity-Selective Magnetization-Prepared Non–Contrast-Enhanced Cerebral MR Angiography at 3 Tesla: Improved Immunity to B0/B1 Inhomogeneity. MRM, 2016; 75(3):1232-1241
6. Taehoon Shin, et al. Identification and Reduction of Image Artifacts in Non–Contrast-Enhanced Velocity-Selective Peripheral Angiography at 3T. MRM, 2016; 76: 466–477.
7. Seiya Kawahara, et al. Non-contrast-enhanced hepatic MR arteriography with balanced steady-state free-precession and time spatial labeling inversion pulse: optimization of the inversion time at 3 Tesla. Acta Radiologica Open, 2015; 4(12): 1-7
Figures